The Reliability of Neuromonitoring to Detect Neurologic Injury during Lateral Interbody Fusion
Yu-Po Lee, Jeffrey Gertsch, William Taylor, David Allison, John Attenello, Kara Nepomuceno, Miguel Nepomuceno and Nitin Bhatia
DOI10.21767/2471-8173.100005
Yu-Po Lee1*, Jeffrey Gertsch2, William Taylor3, David Allison2, John Attenello1, Kara Nepomuceno1, Miguel Nepomuceno1 and Nitin Bhatia1
1Department of Orthopaedic Surgery, University of California, Irvine, CA, USA
2Department of Neurology, University of California, San Diego, CA, USA
3Department of Neurosurgery, University of California, San Diego, CA, USA
- *Corresponding Author:
- Yu-Po Lee
Department of Orthopaedic Surgery, University of California, Irvine, CA, USA
Tel: 7144567012
Email:yupo90025@yahoo.com
Received date: September 15, 2015; Accepted date: october 21, 2015; Published date: october 24,2015
Citation: Lee YP, Gertsch J, Taylor W, et al. The Reliability of Neuromonitoring to Detect Neurologic Injury during Lateral Interbody Fusion. Spine Res. 2015, 1:1.
Abstract
Summary of background data: Lateral interbody fusion is a minimally invasive procedure that is gaining popularity. Hip flexion weakness has been noted postoperatively but the cause of the weakness still remains unknown. The objective of this study is to evaluate if conventional neuromonitoring with electromyography (EMG), somatosensory (SSEP), and motor evoked potentials (MEP) allows surgeons to detect potential nerve injuries that standard EMG monitoring may miss.
Introduction
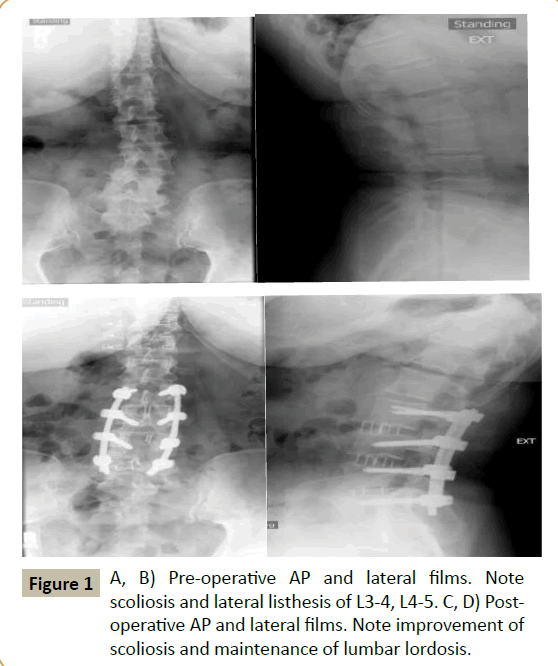
Lateral interbody fusion (LIF) is a minimally invasive approach for fusion of the lumbar spine [1] (Figure 1A-D). Indications for this approach include degenerative scoliosis, spondylolithesis,fractures, infections, and adjacent segment degeneration. Some advantages of this technique compared to traditional open surgery include decreased surgical time, less tissue disruption, a shorter hospital stay, and a faster return to normal activity [2,3].
To perform the lateral interbody fusion, the patient is placed in the lateral decubitus position and the surgeon approaches the spine on the side facing up [1]. With this lateral position, the surgeon can more easily access the spine through the retroperitoneal fat and the psoas muscle. However, potential risks of lateral interbody fusion include injuries to the psoas muscle, exiting ventral nerve roots, and to the genitofemoral nerve [4-6].
Because of these potential risks, many surgeons use electromyographic monitoring (free run and triggered EMGs) while transversing the psoas muscle and docking on the lateral spinal column to minimize detectable injury to the nerves during surgery. However, cases of paresis have been reported in the literature even though the neuromonitoring did not demonstrate any EMG changes [4-6]. In one case report by Houten et al., the authors report two cases where patients underwent L3-4 and L4-5 lateral interbody fusions where patients had hip flexion weakness and paresthesias despite normal neuromonitoring [5]. In both cases, post-operative electromyography and nerve conduction studies showed denervation of the iliopsoas and quadriceps muscles. In another study by Cummock et al., the authors retrospectively reviewed 59 patients who underwent a lateral interbody fusion [6]. Hip flexion weakness and paresthesias were reported at rates of 23.7% and 11.9%, respectively [6]. Hence, questions exist over whether or not standard EMG is adequate enough to detect potential nerve root injuries during LIF procedures.
The objective of this study was to determine the reliability of conventionally used EMG to detect potential nerve injuries and if the addition of somatosensory and motor evoked potentials provides enhanced monitoring ability. This is the first study that compares the EMG neuromonitoring system traditionally used during LIF to conventional neuromonitoring with EMG, somatosensory evoked potentials, and motor evoked potentials.
Methods
The current study is an IRB approved prospective evaluation of patients undergoing LIF between August 2007 and July 2012 at the University of California San Diego.
Patient selection
Patients presenting with degenerative scoliosis, spondylolisthesis, or adjacent segment degeneration with progressive axial and radicular leg pains having failed months of traditional nonoperative treatment were considered candidates for the LIF surgery. Patients were informed of all their surgical options during a preoperative consultation. A description of the LIF procedure was provided and informed consent for surgery was obtained for each patient. Contraindications included severe central canal stenosis, rotatory scoliosis that precluded a lateral approach, auto-fused segments, and moderate to severe spondylolisthesis (≥ grade 3).
This study was conducted in compliance with UC San Diego Human Research Projects Protection Committee guidelines. Following intubation, patients were positioned in the lateral decubitus position with the up side leg flexed at the hip to facilitate relaxation of the psoas muscle. Baseline neurophysiological monitoring signals (neuromuscular junction twitch test, spontaneous EMG (sEMG), triggered EMG (tEMG), transcranial motor evoked potentials (tcMEP), and somatosensory evoked potentials (SSEP)) were obtained. EMG and MEPs were monitored bilaterally in muscles corresponding to the L2-S1 myotome, specifically: vastus medialis, biceps femoris, tibialis anterior, and gastrocnemius muscles. MEPs were recorded prior to docking, and every 5 minutes once the retractor was docked and opened. The tEMG, sEMG, and the neuromuscular junction twitch tests were obtained using the NVM5 neuromonitoring machine (NuVasive Inc., San Diego, California), and sEMG, EEG, TcMEPs, and SSEPs were obtained using the Cascade Elite neuromonitoring system (Cadwell Laboratories Inc, Kennewick, WA 99336).
Patients were prepped and draped in the usual sterile fashion. A standard two-incision approach to the spine was used [1]. Fluoroscopy was used throughout. Following careful dissection to the lateral aspect of the disc space, a guide wire was inserted into the disc and dilators of increasing diameter were inserted to create a path large enough to accommodate the retractor. Nerve elements of the lumbar plexus were localized by triggered EMG generated from a ball tip probe. Once the field was monitored and the retractor was secured to the patient, standard discectomy and interbody fusion was performed in standard fashion.
Clinical outcomes
All consecutive LIF patients who had a minimum 2-year followup were included in the analysis. Data gathered included demographics (age, gender), symptoms and diagnosis, surgical details (levels treated, complications), hospital stay, additional procedures, prospectively collected back and leg pain scores (visual analog scale, VAS) and functional outcome scores (Oswestry disability index, ODI), as well as radiographic fusion assessment. Radiographic review included assessment of fusion at 12 months, 24 months, and/or beyond. Fusion was defined as bridging bone across the interbody site and the absence of motion (>2 mm on flexion/extension radiographs) or lucencies around the instrumentation on plain radiographs. Hip flexion weakness was considered significant if the hip flexion strength decreased by 2 muscle grades or more post-operatively.
Statistical methods
Differences in clinical outcomes scores from preoperative to 2-year postoperative time-points were tested using paired t-tests. All hypothesis testing was performed with a level of significance of 0.05.
Results
Demographics
113 patients were included in the study. This included 50 men and 63 women. Patients averaged 77 years of age (range: 51-98). LIF was performed at a single level in 48 patients, 2-level LIF in 31 patients, and 3-level LIF in 34 patients. In total, LIF was performed in 212 levels. 92% included the use of bone morphogenic protein (BMP), the remainder a mixture of allograft and autograft.
Outcome
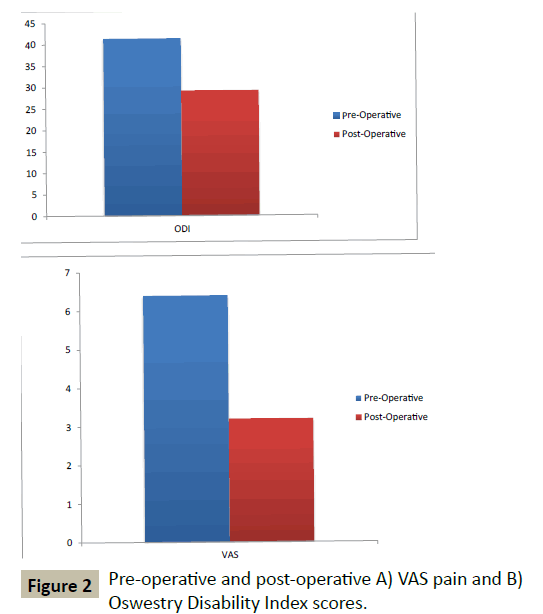
Pain scores (VAS) decreased from 6.4 ± 2.1 pre-operatively to 3.2 ± 2.35 post-operatively at 2 years (p<0.0001) (Figure 2A). Functional scores (ODI) improved by 30% from 41.5 ± 14.6 preoperatively to 29.2 ± 17.5 post-operatively at 2-year follow-up (p<0.0001) (Figure 2B).
Radiographically, spinal fusion across the graft site was achieved in 91% of patients. One patient had a symptomatic pseudarthrosis leading to revision while the other patients who did not achieve complete radiographic fusion were not symptomatic enough to request additional surgery.
Neuromonitoring
Standard EMG was reliable in 110 out of 113 cases (97.3%). In 2 patients, EMG signals were not detectable. One of these patients had Charcot-Marie-Tooth disease and the other had a longstanding history of diabetes and peripheral neuropathy. Both cases were aborted because the nerves were not detectable with twitch testing.
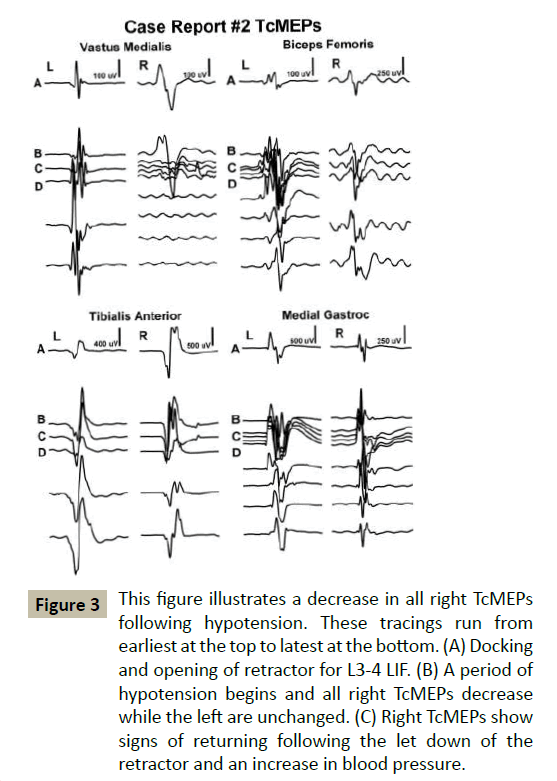
In 3 cases, MEPs signals dropped signifying potential nerve injuries where EMG and SSEPs remained unchanged. In Case #1, MEPs dropped in correlation with a drop in blood pressure (Figure 3). Post-operatively, hip flexion and quadriceps strength were 3/5 on the approach side. Her motor strength had returned to 5/5 by her 12-week follow-up.
Figure 3 This figure illustrates a decrease in all right TcMEPs following hypotension. These tracings run from earliest at the top to latest at the bottom. (A) Docking and opening of retractor for L3-4 LIF. (B) A period of hypotension begins and all right TcMEPs decrease while the left are unchanged. (C) Right TcMEPs show signs of returning following the let down of the retractor and an increase in blood pressure.
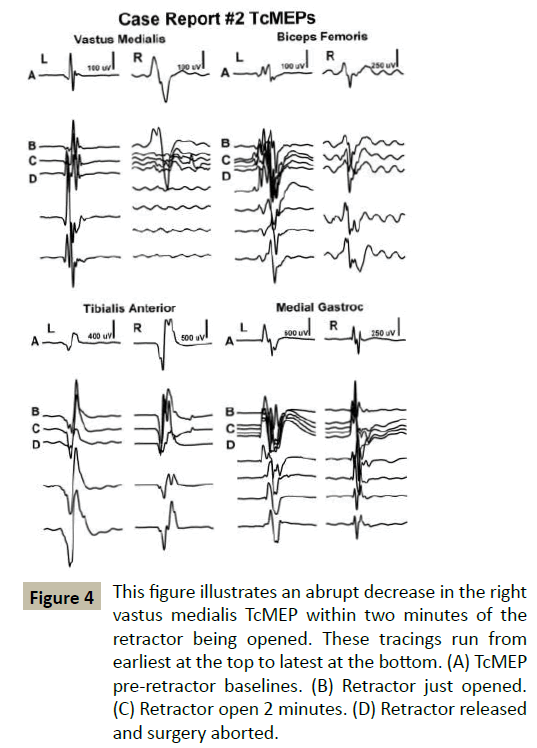
In Case #2, a 59-year-old male consented for right-sided approach L3-4, L4-5 lateral interbody fusion. His past medical history was significant for a stroke and myocardial infarction, vasculopathy, and borderline diabetes. The retractor was docked at L4-5 anterior to nerve elements and opened without incident. During the procedure, TcMEPs showed a drop in signals (Figure 4) while EMG and SSEPs were unchanged. When additional TcMEP testing in the following minute did not show improvement, the retractor was removed and the surgery was aborted. Postoperatively, the patient fired all lower extremity muscles but the right psoas tested 2/5 and the right quadriceps tested 2/5, and reported paresthesia from the hip to lower leg. At 12 weeks following surgery the patient presented with improving strength in his right psoas 3/5, and right quadriceps 3/5, with slight improvement in right leg paresthesia.
Figure 4 This figure illustrates an abrupt decrease in the right vastus medialis TcMEP within two minutes of the retractor being opened. These tracings run from earliest at the top to latest at the bottom. (A) TcMEP pre-retractor baselines. (B) Retractor just opened. (C) Retractor open 2 minutes. (D) Retractor released and surgery aborted.
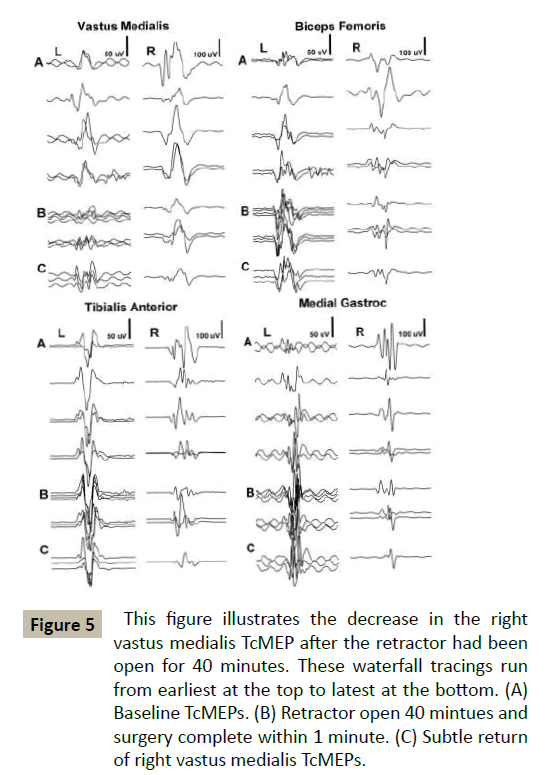
In Case #3 a 73 year old female underwent a LIF at L4-5 for stenosis and spondylolisthesis. Docking of the dilators and retractor occurred without incident. Decrease in tcMEPs was noted at the 40-minute mark (Figure 5). EMG signals remained normal throughout this period. Post-operatively, iliopsoas and quad strength were 3/5. They had returned to 5/5 by her first office visit.
Figure 5 This figure illustrates the decrease in the right vastus medialis TcMEP after the retractor had been open for 40 minutes. These waterfall tracings run from earliest at the top to latest at the bottom. (A) Baseline TcMEPs. (B) Retractor open 40 mintues and surgery complete within 1 minute. (C) Subtle return of right vastus medialis TcMEPs.
Discussion
Lateral interbody fusion (LIF) may be considered a minimally invasive procedure because it satisfies many of the criteria that embody minimally invasive spine surgery. These criteria include less tissue disruption, a shorter hospital stay, and a faster return to normal activity [2,3]. The patients in this study reported a decrease in pain scores from 6.4 pre-operatively to 3.2 postoperatively with a minimum follow-up of 2 years. Oswestry disability scores also improved 30% from 41.5 pre-operatively to 29.2 post-operatively at 2-year follow-up. Radiographic fusion was also achieved in 91%. These results are consistent with other studies on lateral interbody fusion [7-11].
However, iatrogenic nerve injury during the trans-psoas approach is a recognized complication of lateral access surgery. Injuries to the psoas muscle, ventral nerve root, and/or the genitofemoral nerve are potential risks that could occur during surgery. Because of these risks, use of electromyographic monitoring system during surgery has become standard with lateral interbody fuson and the procedure would not be possible without it [2- 7]. However, cases of palsy have been reported in the literature even though the neuromonitoring did not show any injury. Pumberger et al. reported their results in 235 patients [4]. They noted a lumbar plexus deficit in 2.9% of the cases even in the absence of neuromonitoring events. However, they noted a correlation of these neurologic injuries with prolonged surgical time and theorized vascular ischemia as a potential cause of neurologic injury in these cases. In a study by Houten et al., the authors reported two cases where patients underwent L3-4 and L4-5 lateral interbody fusions with post-operative hip flexion weakness and paresthesias despite normal neuromonitoring [5]. Similarly, Cummock et al., the authors retrospectively reviewed 59 patients who underwent lateral interbody fusion [6]. Hip flexion weakness and paresthesias were reported at rates of 23.7% and 11.9%, respectively [6]. Hence, questions exist over how reliable electromyography is in detecting neurologic injury during lateral interbody fusion.
Overall, electromyography is a very reliable neuromonitoring modality to use during lateral interbody fusion. Electromyography neuromonitoring was able to safely guide placement of the retractors in 110 out of 113 cases. In those 110 cases, patients had a motor grade of 4 or greater and hip flexion strength was 5/5 by 6 weeks. In addition, twitch testing with the EMG neuromonitoring system was able to alert the surgical team of 2 patients with severe peripheral nerve abnormalities. One patient had Charcot- Marie-Tooth disease and the other had long-standing diabetes and peripheral neuropathy. Twitch testing in both of these cases did not pick up any signals. In these cases, lateral interbody fusion should not be performed because the EMG neuromonitoring cannot detect the nerves. Testing with standard neuromonitoring with EMG, SSEPs ,and TcMEPs verified these findings.
However, there were 3 cases where patients had clinically significant hip flexion weakness (a decrease in motor strength of 2 motor grades or more) post-operatively and the electromyography neuromonitoring remained silent. Electromyography and somatorsensory evoked potentials in the conventional neuromonitoring system were similarly silent. Hence, this suggests that the EMG neuromonitoring system used during lateral interbody fusion is accurate. However, motor evoked potentials showed a decline in signals during the procedure. The correlation of hip flexion weakness postoperatively with a change in motor evoked potentials suggests that the weakness observed post-operatively may be due to nerve injury that is occurring during the procedure. These injuries have often been attributed to direct mechanical injury brought about by stretch with root traction or direct contact. However,in these 3 cases, the EMG neuromonitoring with both systems remained silent as well as the somatosensory evoked potentials with the conventional neuromontoring system.
There are other reports in the literature of nerve injuries during lateral interbody fusion despite uneventful neuromonitoring with EMG. However, these incidents are rare and it is difficult to link these injuries to a direct cause. The findings of this study raise the question of how the nerve could sustain an injury if the EMG neuromonitoring is silent. It is not unreasonable to postulate that some iatrogenic injuries related to the LIF surgery may have a traction or vascular injury component that evolves over time. In the first case described, the drop in motor evoked potentials correlated with the drop in the systemic blood pressure. In the second case, the patient had a history of a vascular disease. He also had borderline diabetes. All of this suggests disease at the microvascular level. The third patient described had normal signals until the 40-minute mark of retractor opening. Nerve injury from retractor pressure may be correlated with a traction or ischemic injury due to the length of time the retractor was open. This last case correlates well with the study by Pumberger et al., where they noted a correlation of neurologic injuries with prolonged surgical time and also theorized vascular ischemia as a potential cause of neurologic injury in these cases [4]. This theory was also raised by Lykissas et al. who noted a nerve injury rate of 2.3% in their series of 919 patients [12]. If that is the case, decreasing the amount of traction by limiting how much the retractor is opened may be beneficial. Other methods to decrease vascular ischemia on the nerve roots would be to limit how long the retractor is opened for and also to maintain systolic blood pressures above 100 mm Hg during the case. We cannot definitively say that traction or vascular ischemia is the cause of injury to the nerve roots in the cases undetected by EMG. However, we cannot discount this as a possibility since we do not have an answer yet as to why this occurs. Further studies are necessary to better understand this phenomenon.
There are limitations to this study. One of the limitations is that this data was collected at a single institution. The experiences and techniques at this institution may not be representative of other institutions. Another confounding factor is the heterogeneous patient population, with a mix of patient demographics, pathologies, and treatment groups. However, the technique and the outcomes from this study are similar to other studies in the literature and this suggests that the results are probably otherwise similar.
Conclusions
Electromyography is a very reliable monitoring tool during lateral interbody fusion surgery. The addition of twitch testing prior to the procedure can alert the surgical team to any peripheral nerve pathologies that may inhibit the neuromonitoring system. However, there were 3 cases of hip flexion weakness that occurred despite normal electromyography neuromonitoring. Changes in motor evoked potentials were noted specifically in these 3 cases and this suggests that the hip flexion weakness post-operatively may be due to a neurologic injury that is occurring during the procedure. The origin of the nerve injury still has not be elucidated. It has been postulated that the nerve injury may be due to direct trauma from the tubular retractors or from traction. Based on the results of this study, there is some suggestion that the injury may be vascular in origin. If that is the case, decreasing the amount of traction by limiting how much the retractor is opened may be beneficial. Other methods to decrease vascular ischemia on the nerve roots would be to limit how long the retractor is opened for and also to maintain systolic blood pressures above 100 mm Hg during the case.
References
- Ozgur BM, Aryan HE, Pimenta L (2006) Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 26: 435-443.
- Rodgers WB, Gerber EJ, Patterson JR (2011) Intraoperative and early postoperative complications in extreme lateral interbody fusion (XLIF):An analysis of 600 cases. Spine 1: 26-32.
- Isaacs RE, Hyde J, Goodrich JA (2010) A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine 15: 322-330.
- Pumberger M, Hughes AP, Huang RR (2012) Neurologic deficit following lateral lumbar interbody fusion. Eur Spine J 21: 1192-1199.
- Houten JK, Alexandre LC, Nasser R (2011) Nerve injury during the transpsoas approach for lumbar fusion. J Neurosurg Spine 15: 280-284.
- Cummock MD, Vanni S, Levi AD (2011) An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine 15: 11-18.
- Alimi M, Hofstetter CP, Cong GT (2014) Radiological and clinical outcomes following extreme lateral interbody fusion.J Neurosurg Spine 20: 623-635.
- Charosky S, Guigui P, Blamoutier A (2012) Complications and risk factors of primary adult scoliosis surgery: a multicenter study of 306 patients. Spine 15: 693-700.
- Cho SK, Bridwell KH, Lenke LG (2012) Major complications in revision adult deformity surgery: risk factors and clinical outcomes with 2- to 7-year follow-up. Spine 37: 489-500.
- Sansur CA, Reames DL, Smith JS (2010) Morbidity and mortality in the surgical treatment of 10,242 adults with spondylolisthesis. J Neurosurg Spine 13: 589-593.
- Cloyd JM, Acosta FL Jr, Cloyd C (2010) Effects of age on perioperative complications of extensive multilevel thoracolumbar spinal fusion surgery. J Neurosurg Spine 12: 402-408.
- Lykissas MG, Aichmair A, Hughes AP (2012) Nerve injury after lateral lumbar interbody fusion: a review of 919 treated levels with identification of risk factors. Spine J 14: 749-758.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences