Spine Biomechanics: A Review of Current Approaches
Francesco Travascio, Moataz Eltoukhy and Shihab Asfour
DOI10.21767/2471-8173.100004
1Biomechanics Research Laboratory, Department of Industrial Engineering, University of Miami, Coral Gables, FL, 1251 Memorial Drive, McArthur Engineering Building 268, FL 33146, USA
2Sports Medicine and Motion Analysis Laboratory, Department of Kinesiology and Sport Sciences, University of Miami, Coral Gables, FL, 1507 Levante Avenue, Max Orovitz Building 114, FL 33146, USA
- *Corresponding Author:
- Dr. Francesco Travascio
Assistant Professor, College of Engineering, University of Miami 1251 Memorial Drive, 268 McArthur Engineering Building, Coral Gables, FL 33146, USA
Tel: 13052842371- Fax: 13052844040
E-mail: f.travascio@miami.edu - Fax: 13052844040
Received date: August 20,2015 Accepted date: october 20, 2015 Published date: october 24,2015
Abstract
The lumbar spine is a fundamental structure of the human body involved in almost any functional activity. Thus, the investigation of spine pathophysiology is of relevance for many research fields. In particular, research on spine biomechanics may provide important insights on the etiology of diseases affecting spinal tissues. More specifically, spine mechanics and biochemistry can provide crucial information on adverse mechanical loading conditions or unfavorable biochemical environment which can trigger the degeneration of the intervertebral disc, a major structural component of the spine.
Introduction
Lumbar spine offers support to the human body structure, and it is involved in almost all the functional body movements. Accordingly, the investigation of lumbar spine pathophysiology and of approaches for curing spine disorders represents a focal point in many research fields such as ergonomics, kinesiology, sports medicine, and, of course, orthopaedics. In this context, knowledge of spine mechanics (kinetics and kinematics) and biochemistry (tissue homeostasis, degeneration, and restoration) would provide crucial insights on the etiology of spine pathologies, and could suggest strategies for preserving integrity and functionality of this important and yet complex structure of the human body.
Low back pain is a disease of epidemic proportion, whose prevalence in US is only second to common flu [1]. This potentially disabling medical condition is regarded as a serious socioeconomic burden for the Nation [2], and is receiving increasing attention from the research community. The etiology of low back pain and associated radiculopathies are still unclear. However, they have been strongly associated to spine degenerative diseases (intervertebral disc degeneration, zygapophiseal joint osteoarthritis, spondylolisthesis, etc.) [3], which can have either mechanical (abnormal loads, abnormal range of motion, etc.) [4] or mechano-biological origin (impaired tissue nutrition, smoking, ageing, etc.) [5,6]. General aspects of spine pathophysiology are reported in the following section.
Research on spine biomechanics would supply valuable findings for treating and/or preventing the pathologies of this important component of the human body. For instance, an accurate determination of the spine motion and loading conditions are paramount for describing the mechanical environment of the spinal segments. Hereby, current approaches for characterizing spine kinematics are discussed in the third section of this contribution. Also, environmental factors (impaired tissue nutrition, smoking, ageing, etc.) are believed to be directly related to the degradation of spine components. Since spine mechano-biology is very complex and involves a series of intertwined phenomena which are complex to study either in vivo or in in-vitro, computational modeling represents a powerful approach for investigating the etiology of spine pathologies and possible treatments. A brief account on the state of the art of computational models for spine soft tissue components is reported in the fourth section of this review.
Pathophysiology of lumbar spine
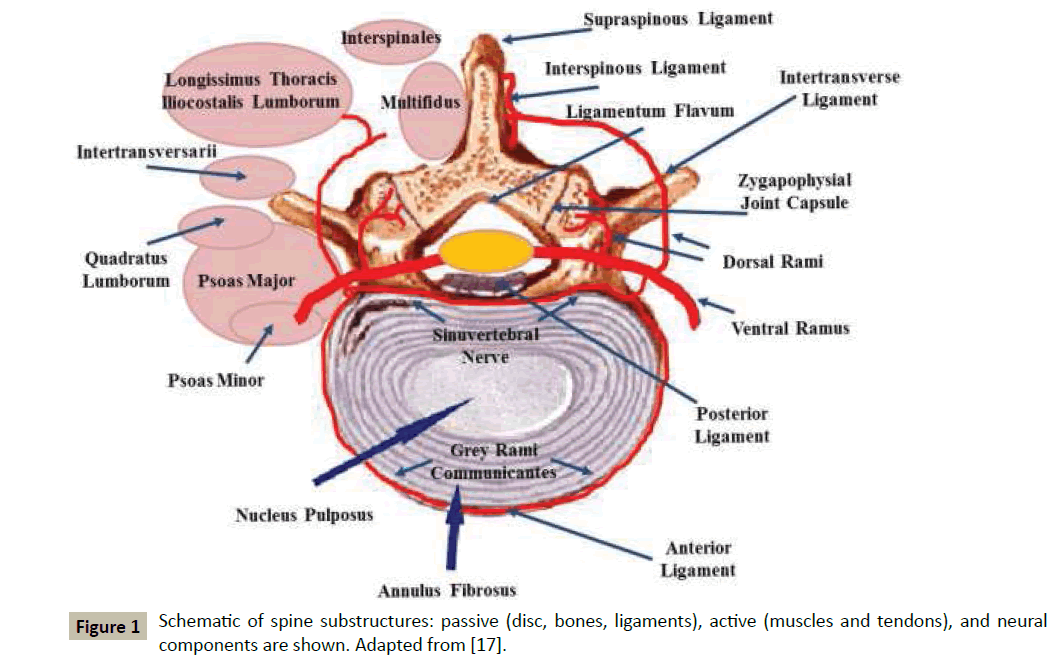
Mobility and stability of the spine are governed by a complex neuro-musculoskeletal system which, in its turn, is usually subdivided in three subsystems: (1) passive, constituted by disc, ligaments, bones, and passive muscles; (2) active, including tendons and active muscles; and (3) neural, accounting for the nervous system and neural components within the passive and active structures, see Figure 1 for a schematic.
The intervertebral disc (IVD) is a soft tissue structure which allows both rotations (flexion-extension, lateral bending, and axial torsion) and translations (cranial-caudal, medial-lateral, and anterior-posterior) of the vertebrae. The neuromuscular system contributes to the stability of the spine. The muscles coordinately act to control movements, balancing the action of gravity, and provide passive elastic tension. A suboptimal motor control, specifically for those activities that are repetitively performed, may cause pain or spine dysfunction.
Usually, muscles as classified based on their hypothesized function as intersegmental or “local”, believed to primarily serve as stabilizers, and multi-segmental or “global”, involved in the production of moments [7-9]. However, debate is still open on which muscles are important stabilizers and which others serve as moment generators. For instance, electromyographic analysis of torso muscles suggested that the more internal muscles transverse abdominis and internal obliques proactively control the stability of the spine [10,11]. Also, an association between lumbar multifidus muscle wasting and symptoms of low back pain has been reported [12], suggesting the involvement of this muscle in spine stabilization. in-vitro biomechanical analyses have predominantly focused on the investigation of local muscles, confirming that they increase the stiffness within the spinal structure, thus playing a critical role for stability [13,14]. However, other authors hypothesize that global muscles may have a more important role in spine stabilization since they act on the whole spine, as opposed to local muscles, which can only act on a few joints [7]. Finally, works from Cholewicki and McGill and Cholewicki and Van Vliet suggest that no single muscle, local or global, possesses a dominant responsibility for lumbar spine stability [15,16].
The mechanisms of muscle recruitments have not been completely elucidated. However, both biomechanical and neurophysiological observations suggested that deep intrinsic muscles control motion at intervertebral level, while multi-segmental ones are involved in the overall control of spine orientation [17]. Spine muscle innervation (mechanoreceptors or load-sensitive endings) provide proprioceptive information necessary for controlling the muscular tone. Besides, nerve endings are also present in passive structures, such as the annulus fibrosus of IVD and in the zygapophyseal joint. The role of these innervations has not been clarified, and it is suggested that such structures provide a feedback on position and movements of passive structures in order to regulate muscle tension to optimize mobility and stability.
As described by Panjabi [18], spine biomechanics depends on the integrity of all three subsystems. In normal conditions, all the musculoskeletal structures interact in a properly coordinated fashion via the neural networks, producing the required movement and, at the same time, providing the necessary stability to the spine. In contrast, when injuries or degenerative processes impair the function of one of those subsystems, their complex and unique balance is disrupted and mechanical loads are distributed in a suboptimal fashion on other spine structures, eventually leading to pain and dysfunctions. More specifically, a dysfunction of any of the three subsystems controlling spine stability would lead to one or more of the following responses in the other subsystems: (1) an immediate compensatory response, resulting in normal function; (2) a long-term adaptation resulting in normal function with an altered spinal stabilizing system; (3) an injury which would lead to overall dysfunction, such as, for example, low back pain.
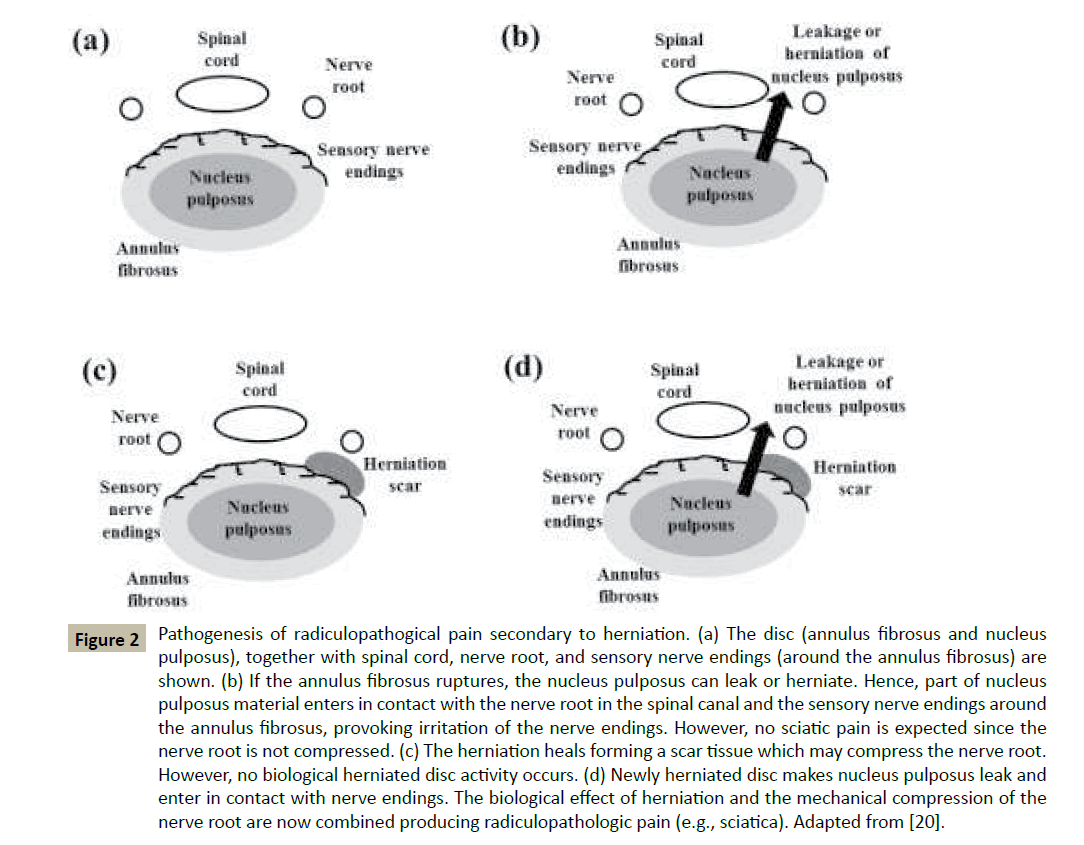
Among all the spinal dysfunctions, degeneration and/or rupture of the IVD is one of the most investigated events. Disc degeneration reduces the mechanical properties of the IVD determining potentially pathogenic loads distributions in the rest of the spine components. For instance, the zygapophysial joint capsule, a synovial joint formed by the superior and the inferior articular processes of two adjacent vertebrae, provides a locking mechanism to spine for resisting to shear translation and axial rotation. Moreover, during bending, this joint bares approximately 20% of the spinal compressive force. However, in case of loss of disc height due to IVD degeneration, the zygapophysial joint may increase its load bearing function up to 70% of the total spinal compressive force [19]. Moreover, in normal physiological conditions, the IVD bear approximately 40% of the torque strength, and the remaining part is attributed to the interspinous ligament. The narrowing of the IVD due to degeneration may reduce the ligament tension, thus decreasing its effectiveness in providing passive stability during rotation or translation [17]. The rupture of disc integrity, as occurring during a process of herniation, has been directly related to the pathogenesis of radiculopathies, such as sciatica. In normal physiological conditions, the nucleus pulposus (NP) of IVD is completely isolated from the environment surrounding the disc. The immediate consequence of the herniation is the contact of NP material with the nerve endings surrounding the disc and the nerve root. This exposure to the external environment elicits an immediate autoimmune response of the tissue. Accordingly, proinflammatory cytokines are expressed at increased rate, causing the formation of scar tissue and generating local pain. However, this biological effect of the disc alone does not cause sciatica.Experimental studies have shown that epidural application of NP material, when in combination of mechanical compression of nerve root, produces sciatic pain. However, no pain is registered when either contact of NP with nerve endings or mechanical compression of nerve root alone occur. It has been hypothesized that, when also mechanical injury (nerve root compression) is superimposed to biological disc effects, concentration of proinflammatory cytokines exacerbates, and radiculopathic pain occurs [20], (Figure 2). Further research is required to elucidate the etiology of this common medical condition, and its diagnosis, treatment, and prevention.
Figure 2 Pathogenesis of radiculopathogical pain secondary to herniation. (a) The disc (annulus fibrosus and nucleus pulposus), together with spinal cord, nerve root, and sensory nerve endings (around the annulus fibrosus) are shown. (b) If the annulus fibrosus ruptures, the nucleus pulposus can leak or herniate. Hence, part of nucleus pulposus material enters in contact with the nerve root in the spinal canal and the sensory nerve endings around the annulus fibrosus, provoking irritation of the nerve endings. However, no sciatic pain is expected since the nerve root is not compressed. (c) The herniation heals forming a scar tissue which may compress the nerve root. However, no biological herniated disc activity occurs. (d) Newly herniated disc makes nucleus pulposus leak and enter in contact with nerve endings. The biological effect of herniation and the mechanical compression of the nerve root are now combined producing radiculopathologic pain (e.g., sciatica). Adapted from [20].
Characterizing in-vivo spine kinematics
It is believed that an altered mechanical environment (e.g., cyclic overloading, chronic limited range of spine motion, etc.) may accelerate the process of IVD degeneration [4]. However, a causal relationship between mechanical overloading and failure of human disc has only been proven in-vitro, due to difficulties in directly measuring spine loads in-vivo. Mainly based on radiographic techniques [21-23], non-invasive approaches for determining in-vivo loads have been developed: by determining relative motion between two including vertebrae, in-vivo levels of disc compression or stretch could be indirectly quantified. Major limitations of such approaches are that, during testing, subject’s motion is constrained, and only specific functional activities of lumbar spine can be monitored. Hence, they are not deployable for investigating spine biomechanics during complex functional activities.
Motion analysis is a widely accepted tool in both clinical and research applications for studying body biomechanics. This approach combines tracking of passive skin-mounted markers to mathematical models for calculating joint kinematics. One of the advantages of this method is that it can be applied for any functional activity of the body with no necessity of motion restrictions. Although most generally used for performing gait analysis, this approach has also been successfully employed for investigating pathological spine conditions [24,25]. However, in these studies, the trunk was considered as a single rigid body. Other studies, aimed at evaluating spine kinematics during walking, utilized multi-segment spine models by applying markers on posterior spinous processes of cervical, thoracic, and lumbar spine [26,27]. In these approaches, an accurate individuation of those spinous processes where markers are supposed to be attached is paramount for the precision of the spine kinematics measurements. Hence, the localization of lumbar posterior spinous processes may represent a significant technical challenge when subjects involved in the analysis have a body structure characterized by a significant presence of fat tissue on the lower back.
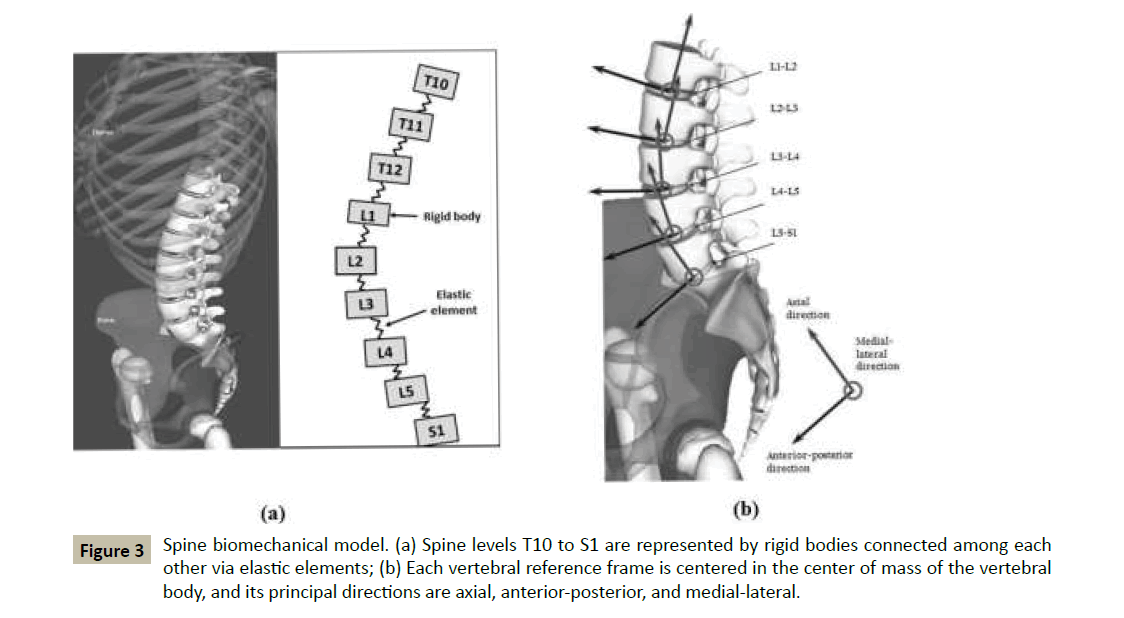
An alternative approach to predict in-vivo loads on lumbar spine, combining an analytical biomechanical framework to motion capture, has recently been proposed [28,29]. More specifically, a biomechanical model schematizes the spine as a kinematic chain of rigid bodies representing lumbar and thoracic vertebrae (from T10 to S1), which are connected among each other by intervertebral discs, see Figure 3. Motion analysis of skinmounted markers on subject’s pelvis and thorax provide input data for the model to calculate relative motion among vertebrae. These data are deployed into an inverse kinematic analysis for determining forces and moments acting on lumbar vertebrae. In principle, this tool would be able to predict lumbar spine loads for any functional activity of the human body circumventing those technical challenges associated to the experimental approaches above discussed.
Figure 3 Spine biomechanical model. (a) Spine levels T10 to S1 are represented by rigid bodies connected among each other via elastic elements; (b) Each vertebral reference frame is centered in the center of mass of the vertebral body, and its principal directions are axial, anterior-posterior, and medial-lateral.
Modeling the biomechanics of soft tissues in the spine
The IVD is the largest soft tissue structure in the spine, providing load support and spinal flexibility. Due to its crucial functional and structural roles in spinal biomechanics, IVD health is intimately related to the health of the whole spine: disc degradation has been associated with several spine pathologies (e.g., low back pain, stenosis, spine instability, etc.) [3,30,31]. The continuous presence of a compressive load on the IVD may compromise the integrity of the extracellular matrix (ECM) of the disc. Therefore, in order to accomplish with its structural function, the IVD undergoes a continuous turnover of its ECM. This homeostatic process is regulated by a delicate balance between catabolic and anabolic cellular processes: while, through catabolism, damaged or old components of the ECM are disposed, during the anabolic cellular activity, matrix structure is either repaired or replaced. Disruption of the homeostatic balance, due to insufficient anabolic activity or excessive catabolism, leads to the degeneration of the IVD [32]. To date, the precise mechanisms causing the alteration of IVD homeostasis are not completely understood yet, and it is believed that the etiology of disc degeneration is multifactorial including, among other factors, genetic influence, aging, adverse mechanical environment, and insufficient nutritional supply [4,5,33,34].
In vivo and in vitro studies have contributed to shed light on the mechanisms associated to disc physiology and pathology. However, such experimental approaches present limitations in accurately quantifying the spatial and temporal variations of internal stresses and strains within tissue, transport phenomena, chemical reactions, and parameters affecting them. As an alternative, theoretical frameworks and computational models can provide crucial insights into how IVD functions and fails. Accordingly, during the past decades, numerous numerical tools have been developed and deployed to investigate IVD functional biomechanics [35].
For instance, several studies investigated IVD nourishment and metabolism. The IVD is the largest avascular structure in the human body, receiving nutrients from the vascular network surrounding the tissue at cartilage endplates (CEP), and around the lateral surface of the annulus fibrosus (AF) [36-38]. Numerical models showed that nutrients (i.e., oxygen and glucose) concentrations decrease with increasing distance from disc boundaries to critical values at tissue mid-height near the NP-AF boundary [39-41]. The same studies also showed that such low levels of nutrients further decrease when, due to tissue degradation, the porosity of the endplate reduces. These alterations in the nutrient distribution are important since insufficient nutritional supply can adversely affect the ability of disc cells to adequately synthesize and maintain ECM, ultimately leading to IVD degeneration [42].
In contrast to small solutes (e.g., oxygen, glucose, etc.), larger molecules (e.g., cytokines regulating cellular processes, PG composing the ECM, etc.) have a limited penetration into the disc [43]. In this case, convective transport due to interstitial fluid redistribution upon physiologic mechanical loading of IVD enhances transport of these large molecular weight solutes. Through computational modeling, it has been estimated that the net effect convective transport for large solutes over a full diurnal cycle is about 30% larger in solute penetration compared to diffusion alone [43].
Several computational studies have also attempted to account for the presence of cells in IVD by formulating cell viability criteria based on available experimental data. Usually in these models, cell death initiates as glucose levels fall below experimentally determined threshold levels [44,45]. The results of studies deploying these computational tools suggested that, when tissue degeneration is driven by impaired nutritional supply or calcification of CEP area, cell death appears first in the central region of the NP (where glucose concentration is the lowest), and subsequently extends towards the inner the AF and to the CEP as degenerative conditions exacerbate [46-51].
Recently, several research groups have tried to address disc degeneration by developing strategies which aim at stimulating the anabolic activity of disc cells. Such approaches include growth factor therapies, which are treatments based on the exogenous administration of agents (growth factors) stimulating cells in producing ECM components [52]. Several growth factors are known to up-regulate anabolism in IVD (e.g., TGF-?, IGF-1, BMPs, etc.) [32]. In particular, it has been reported that IGF-1 stimulates the production of proteoglycans (PG) in the NP cells of the IVD [53,54]. Therefore, the exogenous administration of IGF-1 has been highly regarded as a potential therapy for disc degeneration. Unfortunately, preliminary studies on intradiscal injection of IGF-1 in degenerated IVD of rabbit did not produce satisfactory results [55]. Results obtained via computational models for IVD homeostasis suggest that this was likely due to the dosage, time sequence, and composition of the administered bolus containing the growth factor [56,57].
The IGF-mediated biosynthesis of PG is the product of an intercellular signaling cascade initiated when IGF-1 binds to IGF-specific cell surface receptors [58-60]. Hence, the efficiency of this biosynthetic mechanism depends on how many IGF-1 molecules bind to cell receptors in the tissue. Because of this dose-dependent response of IVD cells to IGF-1 stimulation, knowledge and control of the distribution of this growth factor in the disc is crucial for the success of the therapy. This is not an easy task, since several issues should be considered. For instance, the half-life of IGF-1 in IVD may represent a limiting factor [52]: during the administration, IGF-1 may degrade before reaching the target cells to be stimulated. Also, IGF-1 increases the nutritional demand of IVD cells by promoting cellular proliferation [61], and by enhancing cellular metabolism due to increased PG production [56]. Hence, since impaired nutrition represents a leading cause to disc degeneration [5], IGF-1 administration might turn out to be detrimental for the health of the IVD.
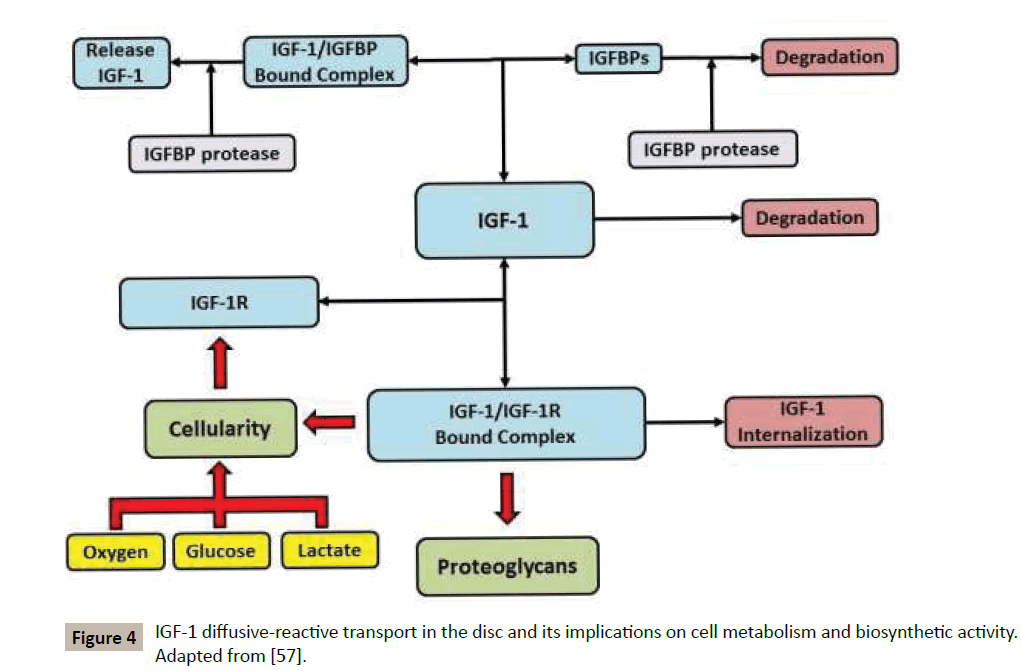
A model for the complex interactions of IGF-1 with IVD cells and ECM has been proposed [57], see Figure 4 for a schematic. In the ECM of IVD, IGF-1 competitively reacts with IGF binding proteins (IGFBPs) and IGF-1 cell surface receptors (IGF-1R) [60]. The first of these binding reactions leads to the formation of a bound complex (IGF-1/IGFBP), which can reversibly dissociate into its constituents, or be degraded by IGFBP-protease to free IGF- 1 [62]. The interaction between IGF-1 and IGF-1R, leading to the formation of bound cell receptors (IGF-1/IGF-1R), is also reversible, and upregulates both PG production and cell proliferation [54,61].
Alternatively, upon formation of the bound complex IGF-1/IGF-1R, the growth factor can be internalized (eliminated) by disc cells without any stimulation of either ECM biosynthetic rate or cell proliferation rate [58]. In addition, both degradation of IGF-1 and enzymatic degradation of binding proteins via IGFBP-protease are considered. The model has been deployed for quantifying the effect of ageing and tobacco smoking on the disruption of the health of the IVD [63,64], and to investigate potential growth factor therapies for disc restoration [57,65]. Numerical results indicate that an adequate IVD anabolism requires both sufficient IGF-1 availability and cellularity, the latter conditional to sufficient levels of nutrients for allowing cells to survive and synthetize ECM. In a normal physiological scenario, these conditions are met. In contrast, in pathological scenarios characterized by nutrients deprivation, ageing, or smoking, cell death occurs and causes a suboptimal PG distribution. Moreover, the systemic administration of exogenous IGF-1 for increasing the anabolic activity of IVD cells is only beneficial in those disc regions receiving sufficient nutritional supply (i.e., the most external regions in proximity with the vascular network), while its presence exacerbate tissue degeneration in malnourished regions. Taken together, these results suggest that an adequate nutritional supply is paramount for ensuring disc health, and should be attained for a successful outcome of growth factorbased therapies for IVD degeneration.
Conclusions
Spine pathophysiology is complex, and dysfunctions can occur when the integrity of its passive, active, or neural components is compromised. Among all the spinal dysfunctions, degeneration of the IVD is one of the most investigated events, and its etiology is still unclear. Unfavorable mechanical loading conditions and impaired nutrition are considered among the potentially responsible for the degradation of the IVD. Accordingly, research pathways have been delineated for investigating the both the mechanical (loading conditions) and the chemical (nutrition supply) environment of the IVD.
Several techniques have been developed for measuring lumbar spine kinematics. Imaging techniques are able to calculate spine motion, but only for restricted activities. In contrast, motion capture methods can determine spine kinetics and kinematics for unrestricted body motion, making these approaches suitable for investigating disc mechanical environment during spine functional activities.
The homeostasis of the intervertebral disc is regulated by a delicate balance between anabolic and catabolic activities of disc cells which is very difficult to investigate in vivo or in vitro experiments. Hence, mathematical modeling represents a powerful approach for acquiring new knowledge on the mechanisms regulating disc physiology and pathology. Several factors can disrupt disc homeostasis, and insufficient nutritional supply to IVD tissue, low cellularity, or reduced anabolic activity of disc cells are crucial ones.
Acknowledgments
Study supported by funds donated to Biomechanics Research Group of the University of Miami.
References
- Long D M, Bendebba M, Torgerson W S, Warren S (1996) Persistent Back Pain and Sciatica in the United States: Patient Characteristics. Journal of Spinal Disorders 9: 40-58.
- Nih (1997) Research on Low Back Pain and Common Spinal Disorders. NIH Guide 26
- Arbit E, Pannullo S, (2001) Lumbar Stenosis: A Clinical Review. Clinical Orthopaedics and Related Research 384: 137-143.
- Stokes A F, Iatridis J C (2004) Mechanical Conditions That Accelerate Intervertebral Disc Degeneration: Overload Versus Immobilization. Spine 29: 2724-2732.
- Urban J P, Smith S, Fairbank J C(2004) Nutrition of the Intervertebral Disc. Spine 29: 2700-2709.
- Okuda S, Myoui A, Ariga K, Nakase T, Yonenobu K, et al. (2001) Mechanisms of Age-Related Decline in Insulin-Like Growth Factor-I Dependent Proteoglycan Synthesis in Rat Intervertebral Disc Cells. Spine 26: 2421-2426.
- Panjabi M M, Abumi K, Duranceau J, Oxland T R, (1989) Spinal Stability and Intersegmental Muscle Forces: A Biomechanical Model. Spine 14: 194-200.
- Bergmark A (1989) Stability of the Lumbar Spine. A Study in Mechanical Engineering Acta Orthopaedica Scandinavica 60: 1-54.
- Richardson C, Jull G, Hodges P, Hides J,Panjabi M M (1999)Therapeutic Exercise for Spinal Segmental Stabilization in Low Back Pain: Scientific Basis and Clinical Approach, Churchill Livingstone, Edinburgh.
- Hodges P, Richardson C A(1997) Feedforward Contraction of Transverse Abdominis Is Not Influenced by the Direction of Arm Movement. Experimental Brain Research 114: 362-370.
- Hodges P W, Richardson C A(1998) Delayed Postural Contraction of Transversus Abdominis in Low Back Pain Associated with Movement of the Lower Limb. Journal of Spinal Disorders & Techniques 11: 46.
- Hides J, Stokes M J, Saide M J, Jull G, Cooper D H(1994) Evidence of Lumbar Multifidus Muscle Wasting Ipsilateral to Symptoms in Patients with Acute/Subacute Low Back Pain. Spine 19: 165-172.
- Wilke H J, Wolf S, Claes L, Arand M, Wiesend A, (1995) Stability Increase of the Lumbar Spine with Different Muscle Groups: A Biomechanical in Vitro Study. Spine 20: 192-197.
- Crisco J J, Panjabi M M(1991) The Intersegmental and Multisegmental Muscles of the Lumbar Spine. A Biomechanical Model Comparing Lateral Stabilizing Potential Spine 16: 793-799.
- Cholewicki J, Mcgill S. M (1996) "Mechanical Stability of the in Vivo Lumbar Spine: Implications for Injury and Chronic Low Back Pain. Clinical Biomechanics 11: 1-15.
- Cholewicki J, Vanviliet J (2002) Relative Contribution of Trunk Muscles to the Stability of the Lumbar Spine During Isometric Exertions. Clinical Biomechanics 17: 99-105.
- Holm A M, Holm S H(2004) The Lumbar Spine. Lippincott Williams and Wilkins, Philadelphia, PA, Biomechanical Condiserations in Disc Degeneration.
- Panjabi M M (1992) The Stabilizing System of the Spine. Part I. Function, Dysfunction, Adaptation, and Enhancement. Journal of Spinal Disorders 5: 383-389.
- Dunlop R B, Adams M A, Hutton W C (1984) Disc Space Narrowing and the Lumbar Facet Joints. Journal of Bone and Joint Surgery British 66: 706-710.
- Olmarker K, Myers R R, Kikuchi K, Rydeyik B(2004) The Lumbar Spine, Lippincott Williams and Wilkins, Philadelphia, PA, Pathophysiology of Nerve Root Pain in Disc Herniation and Spinal Stenosis.
- Wong K W, Luk K D, Leong J C , Wong S F, Wong K K (2006) Continuous Dynamic Spinal Motion Analysis. Spine 31: 414-419.
- Ochia R S, Inoue N, Renner S, Lorenz E P, Lim T H, et al. (2006) Three-Dimensional in Vivo Measurement of Lumbar Spine Segmental Motion. Spine 31: 2073-2078.
- Li G, Wang S, Passias P, XiaQ, Li G, Wood K(2009) Segmental in Vivo Vertebral Motion During Functional Human Lumbar Spine Activities. European Spine Journal 18: 1013-1021.
- Engsberg J R, Bridwell K H, Ritenbach A K, Uhrich M L, Baldus C, et al.(2001) Preoperative Gait Comparisons between Adults Undergoing Long Spinal Deformity Fusion Surgery (Thoracic to L4, L5, or Sacrum) and Controls. Spine 26: 2020-2028.
- Engsberg J R, Bridwell K H, Wagner J M, Uhrich ML, Blanke K, et al. (2003) Gait Changes as the Result of Deformity Reconstruction Surgery in a Group of Adults with Lumbar Scoliosis. Spine 28: 1836-1844.
- Syczewska M, Oberg T, Karlsson D(1999) Segmental Movements of the Spine During Treadmill Walking with Normal Speed. Clinical Biomechanics 14: 384-388.
- Konz R J, Fatone S, Stine R L, Ganju A, Gard S A, et al. (2006) A Kinematic Model to Assess Spinal Motion During Walking. Spine 31: 898-906.
- Eltoukhy M, Ziff M, Elmasry S, Travascio F, Asfour S(2014) A Novel Approach for Predicting in-Vivo Lumbar Spine Loads and Kinematics Based on Motion Analysis," eds., Boston, MA, pp.
- Eltoukhy M,Travascio F, Asfour S, Elmasry S, Heredia-Vargas H, et al.(2015) Examination of a Lumbar Spine Biomechanical Model for Assessing Axial Compression, Shear, and Bending Moment Using Selected Olympic Lifts," Journal of Orthopaedics, doi:10.1016/j.jor.2015.04.002(pp.
- Katz J N(2006) Lumbar Disc Disorders and Low-Back Pain: Socioeconomic Factors and Consequences. The Journal of Bone and Joint Surgery 88: 21-24.
- Fujiwara A, Tamai K, An H S, Kurihashi A, Lim T H, et al. (2000) The Relationship between Disc Degeneration, Facet Joint Osteoarthritis, and Stability of the Degenerative Lumbar Spine. Journal of Spinal Disorders 13: 444-450.
- Masuda K, An H S(2004) Growth Factors and the Intervertebral Disc. The Spine Journal 4: 330-340.
- Buckwalter J A(1995) Aging and Degeneration of the Human Intervertebral Disc. Spine 20: 1307-1314.
- Battie M C, Videman T, Parent E(2004) Lumbar Disc Degeneration: Epidemiology and Genetic Influence. Spine 29: 2679-2690.
- Schmidt H, Galbusera F, Rohlmann A, Shirazi-Adl A(2013) What Have We Learned from Finite Element Model Studies of Lumbar Intervertebral Discs in the Past Four Decades. Journal of Biomechanics 46: 2342-2355.
- Holm S, Maroudas A, Urban J P, Selstam G, Nachemson A(1981) Nutrition of the Intervertebral Disc: Solute Transport and Metabolism. Connective Tissue Research 8: 101-109.
- Nachemson A, Lewin T, Maroudas A, Freeman M A(1970) In Vitro Diffusion of Dye through the End-Plates and the Annulus Fibrosus of Human Inter-Vertebral Disc. Acta Orthopaedica Scandinavica 41: 589-607.
- Ogata K, Whiteside L A(1981) Nutritional Pathways of the Intervertebral Disc. An Experimental Study Using Hydrogen Washout Technique. Spine 6: 211-216.
- Selard E, Shirazi-Adl A, Urban J P(2003) Finite Element Study of Nutrient Diffusion in the Human Intervertebral Disc. Spine 28: 1945-1953.
- Soukane D M, Shirazi-Adl A, Urban J P (2007) Computation of Coupled Diffusion of Oxygen, Glucose and Lactic Acid in an Intervertebral Disc. Journal of Biomechanics 40: 2645-2654.
- Malandrino A, Noailly J, Lacroix D(2011) The Effect of Sustained Compression on Oxygen Metabolic Transport in the Intervertebral Disc Decreases with Degenerative Changes. PLOS Computational Biology 7: 1-12.
- Ishihara H, Urban J P(1999) Effects of Low Oxygen Concentrations and Metabolic Inhibitors on Proteoglycan and Protein Synthesis Rates in the Intervertebral Disc. Journal of Orthopaedic Research 17: 829-835.
- Ferguson S J, Ito K, Nolte L P (2004) Fluid Flow and Convective Transport of Solutes within the Intervertebral Disc. Journal of Biomechanics 37: 213-221.
- Bibby S R, Urban J P(2004) Effect of Nutrient Deprivation on the Viability of Intervertebral Disc Cells. European Spine Journal 13: 695-701.
- Bibby S R, Jones D A, Ripley R M, Urban J P(2005) Metabolism of the Intervertebral Disc: Effects of Low Levels of Oxygen, Glucose, and Ph on Rates of Energy Metabolism of Bovine Nucleus Pulposus Cells. Spine 30: 487-496.
- Shirazi-Adl A, Taheri M, Urban J P(2010) Analysis of Cell Viability Inintervertebral Disc — Effect of Endplate Permeability on Cell Population. Journal of Biomechanics 43: 1330-1336.
- Galbusera F, Mietsch A, Schmidt H, Wilke H J, Wilke C N(2013) Effect of Intervertebral Disc Degeneration on Disc Cell Viability: A Numerical Investigation. Computer Methods in Biomechanics and Biomedical Engineering 16: 328-337.
- Zhu Q, Jackson A R,Gu W Y(2012) Cell Viability in Intervertebral Disc under Various Nutritional and Dynamic Loading Conditions: 3d Finite Element Analysis. Journal of Biomechanics 45: 2769-2777.
- Zhu Q, Gao X, Gu W Y(2014) Temporal Changes of Mechanical Signals and Extracellular Composition in Human Intervertebral Disc During Degenerative Progression. Journal of Biomechanics 47: 3734-3743.
- Gu W Y, Zhu Q, Gao X, Brown M D(2014) Simulation of the Progression of Intervertebral Disc Degeneration Due to Decreased Nutrition Supply. Spine 39: 1411-1417.
- Malandrino A, Noailly J Lacroix D(2014) Numerical Exploration of the Combined Effect of Nutrient Supply, Tissue Condition and Deformation in the Intervertebral Disc. Journal of Biomechanics 47: 1520-1525.
- Masuda K, An H(2008) Motion Preservation Surgery of the Spine — Advanced Techniques and Controversies, Elsevier, Amsterdam, Growth Factors for Intervertebral Disc Regeneration.
- Thompson J P, Oegema T R, Bradford D S(1991) Stimulation of Mature Canine Intervertebral Disc by Growth Factors. Spine 16: 253-260.
- Osada R, Ohshima H, Ishihara H, Yudoh K, Sakai K, et al. (1996) Autocrine/Paracrine Mechanism of Insulin-Like Growth Factor-1 Secretion, and the Effect of Insulin-Like Growth Factor-1 on Proteoglycan Synthesis in Bovine Intervertebral Discs. Journal of Orthopaedic Research 14: 690-699.
- Walsh A J , Bradford D S, LotzJ C(2004) In Vivo Growth Factor Treatment of Degenerated Intervertebral Discs. Spine 29: 156-163.
- Huang C Y, Travascio F, Gu W Y(2012) Quantitative Analysis of Exogenous Igf-1 Administration of Intervertebral Disc through Intradiscal Injection. Journal of Biomechanics 45: 1149-1155.
- Travascio F, Elmasry S, Asfour S (2014) Modeling the Role of Igf-1 on Extracellular Matrix Biosynthesis and Cellularity in Intervertebral Disc," Journal of Biomechanics, 10.1016/j.jbiomech.2014.04.046(pp.
- Lauffenburger D A, Linderman J J(1993) Receptors: Models for Binding, Trafficking and Signaling. , Oxford University Press, New York.
- Foulstone E, Prince S, Zaccheo O, Burns J L, Harper J, et al. (2005) Insulin-Like Growth Factor Ligands, Receptors, and Binding Proteins in Cancer. Journal of Pathology 205: 145-153.
- Paye J M, Forsten-Williams K(2006) Regulation of Insulin-Like Growth Factor-I (Igf-I) Delivery by Igf Binding Proteins and Receptors. Annals of Biomedical Engineering 34: 618-632.
- Pratsinis H, Kletsas D(2007) Pdgf, Bfgf and Igf-I Stimulate the Proliferation of Intervertebral Disc Cells in Vitro Via the Activation of the Erk and Akt Signaling Pathways. European Spine Journal 16: 1858-1866.
- Durai R, Yang W, Gupta S, Seifalian A M, Winslet M C(2005) The Role of the Insulin-Like Growth Factor System in Colorectal Cancer: Review of Current Knowledge. International Journal of Colorectal Disease 20: 203-220.
- Asfour S, Travascio F, Elmasry S, De Rivero Vaccari J P (2015) A Computational Analysis on the Implications of Age-Related Changes in the Expression of Cellular Signals on the Role of Igf-1 in Intervertebral Disc Homeostasis. Journal of Biomechanics 48: 332-339.
- Elmasry S, Asfour S, De Rivero Vaccari J P, Travascio F, (2015) Effects of Tobacco Smoking on the Degeneration of the Intervertebral Disc: A Finite Element Study," PloS one, In Press(pp.
- Travascio F, Elmasry S, Asfour S(2014) Barriers to Growth Factor Therapy for Intervertebral Disc Degeneration: A Quantitative Analysis on the Effectiveness of Exogenous Igf-1 Administration," eds., Boston, MA, pp.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences