Predictive Value of Native Computed Tomography in Low Energy Vertebra Fracture Risk Assessment
DOI10.21767/2471-8173.100009
Andrey Bokov1, Alexander Aleynik1, Marina Rasteryaeva2, Marina Kutlaeva2, Sergey Mlyavykh1 and David G. Anderson3*
Department of Neurosurgery, Privolzhski Federal Medical Research Center, USA
Department of Radiology, Privolzhski Federal Medical Research Center, USA
Departments of Orthopaedic Surgery and Neurological Surgery Thomas Jefferson University and Rothman
- *Corresponding Author:
- David G. Anderson
Professor, Department of Orthopaedic Surgery, Neurological Surgery, Thomas Jefferson University, The Rothman Institute, 925 Chestnut St, 5th floor, PA, 19107, Philadelphia, USA
Tel: 434825-8916
Fax: 215503-0580
E-mail: greg.anderson@rothmaninstitute.com
Received date: August 28, 2015; Accepted date: December 25, 2015; Published date: December 29, 2015
Citation: Bokov A, Aleynik A, Rasteryaeva M, et al. Predictive Value of Native Computed Tomography in Low Energy Vertebra Fracture Risk Assessment . Spine Res. 2015, 2:1. doi:10.21767/2471-8173.100009
Abstract
http://spine.imedpub.com/spine.imedpub.com/ (CT) can be used to accurately determine bone density in Hounsfield units (HU), the use of CT as a predictive tool has not been conclusively demonstrated in relation to low energy vertebra compression fracture (VCF). The aim of this study was to define the CT parameters that could be used to predict the risk of VCF.
Keywords
Computed tomography; Hounsfield units; Bone minimal density; Vertebral compression fracture
Introduction
Vertebral compression fractures (VCF) are a commonly encountered clinical condition in the older adult population and often require nonoperative and operative therapies. It has been estimated that 40% of women will experience this pathology within their lifetime [1-3]. Following an initial VCF, the odds of recurrent VCF within a decade is over 25% [4]. Although both genders are affected by VCF, the incidence is noticeably higher in females [5-7]. VCF leads to a variety of secondary problems including progressive spinal deformity, decreased lung capacity, and reduced gastrointestinal function [8-10].
Dual emission x-ray absorptiometry (DEXA) is frequently used to assess bone mineral density of the lumbar spine, hip, wrist or calcaneus and has been used widely as a diagnostic tool in the surveillance of osteoporosis. Unfortunately, the sensitivity of DEXA in predicting fracture risk has been shown to be relatively poor, leading some to suggest the need for additional diagnostic studies that more accurately predict fracture risk [11-13]. Computed tomography (CT) is a commonly employed diagnostic modality useful in the workup of a variety of spinal conditions. CT data is capable of accurately defining bone density using Hounsfield units (HU). The HU scale represents the relative radiodensity of a body tissue according to a calibrated graylevel scale, based on the values for air (−1000 HU) and water (0 HU); this scale is slightly non-linear [14]. The use of the HU scale has been utilized to measure the likelihood of success in dental implants, a procedure which relies on a stable bone-implant interface [15,16]. Some have suggested that CT measures could play a useful role in identifying patients at risk for low energy VCF [17,18]. It was clearly defined that both phantomless quantitative computed tomography and simple ROI attenuation measurements are effective for bone density screening, however the opportunity for VCF prediction was not investigated [19]. In this study, we hypothesized that a threshold of bone density in HU could be identified, below which the risk of VCF would be significantly increased.
Materials and Methods
One hundred consecutive patients older than 40 years were enrolled in this study between August and December 2012. Patients were selected for study inclusion if they underwent a CT scan of the lumbar spine as a part of the medical work up for symptoms of axial back pain. Patients with a history of high energy trauma or oncologic lesions were excluded from study participation.
The CT scans were performed from the T10-L5 levels using a single CT scanner (Aquilion 32, Toshiba Corporation). The scans utilized a slice thickness was 0.5 mm, covering a scan area of 50 cm. The scan parameters included: tube voltage 120 kV, tube current 300 mA, auto mAs range 180-400; 1.0 sec/3.0 mm/0.5 × 32, helicalpitch 21.0. Integrated software was utilized for calculations of bone density (Vitrea Version 5.2.497.5523) incorporating a window width/window level ratio of 2000/500.
Measurements of bone density of the cancellous portions of the vertebral bodies without presence of VCF were obtained in HU at the level of L2 or L3 in the sagittal, axial and coronal planes. Measurements in the axial plane were taken at the level of the middle of the pedicles. Measurements in the sagittal and coronal planes were taken along the geometric center of vertebra body. Oval-shape trabecular bone samples were selected using the maximal achievable diameters without traversing into cortical bone to calculate bone density in each plane. All cases with VCF were inspected by two certified radiologists using Genant visual semi-quantitative method [20] and were categorized as singlelevel or multi-level (greater than one level of VCF). Patients with degenerative changes in the spinal segments defined as loss of more than 50% of the disc height and associated endplate sclerosis were identified.
Statistical Analysis
A power analysis was performed using a data subset to estimate sample size by the Monte-Carlo method with 2000 simulations. The interaction of bone density in HU, the presence of singlelevel or multi-level VCF and the presence of degenerative changes were assessed using logistic regression analysis.
Results
The study included 63 females (63%) and 37 males (37%) with a mean age of 58 years (42-89 years). The age and bone density measurements are shown in Table 1.
| n=100 | Mean (mean ± Standard error of mean) |
Standard deviation | Maximum | Minimum |
|---|---|---|---|---|
| Patient age | 58.18 ± 0.95 | 9.53 | 89 | 42 |
| Bone density axial HU | 102.01 ± 4.87 | 48.75 | 225 | -28 |
| Bone density sagittal HU | 113.50 ± 5.03 | 50.39 | 227 | -20 |
| Bone density coronal HU | 109.59 ± 5.17 | 51.70 | 212 | -22 |
| Mean bone densityHU | 108.37 ± 4.95 | 49.56 | 212 | -17 |
Table 1: Age and bone density measurements for the study cohort.
The mean bone density was calculated from the axial, sagittal and coronal measurements of each vertebral segment and was used in the logistic regression analysis.
The frequency of VCF and degenerative changes is shown in Table 2.
| n=100 | Detected VCF | Multi-level VCF | Degenerative changes |
|---|---|---|---|
| N of patients | 27 | 18 | 52 |
Table 2: VCFs and degenerative changes.
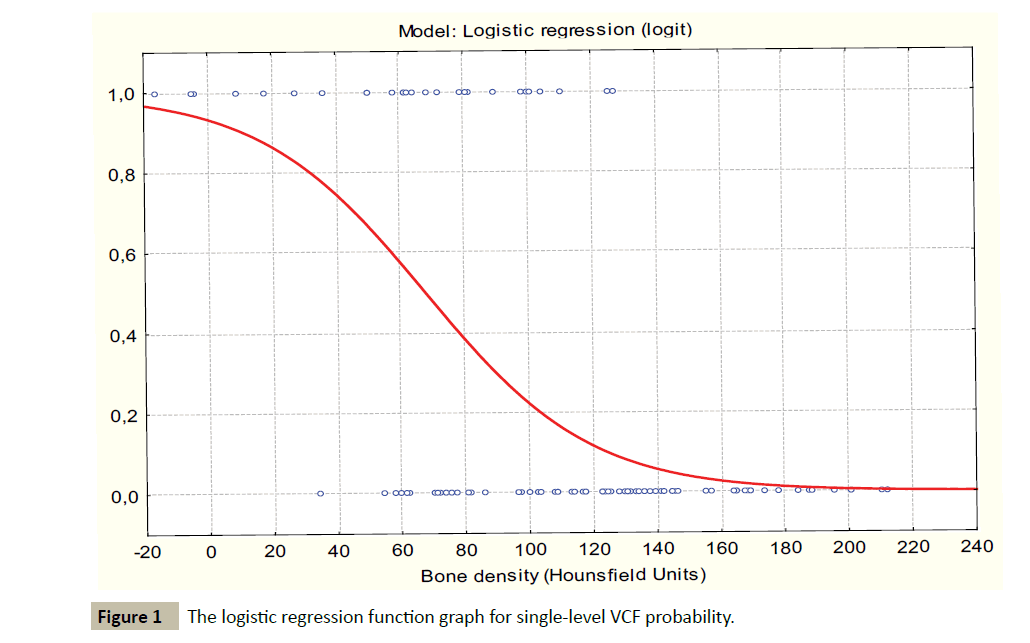
The logistic regression analysis demonstrated a strong positive correlation between the rate of single level VCF and decreasing bone density. The parameters of the logistic regression model were ÃÆÃÂÃâââ¬â¢0=2.6254, p=0.0013; B1= -00387, p<0.0001. Goodness of fit: Chi-square=37.1180; p<0.0001, percent of correctly predicted 81% (Figure 1).
The estimated logistic function: y=e2.6254-0.3872*x/(1+e2.6254- 0.3872*x)
No fractures were observed in cases where the bone density was above 150 HU. The logistic regression model demonstrated a critical point of 101.78 HU associated with the significant increase in rate of single-level VCF (critical point associated with VCF probability growth acceleration was detected using derivatives of logistic regression function). The estimated logistic regression for this value was B0=0, p=1 (not significant); B1=2.024382, p=0.00014; odds ratio(OR)=7.57; 95% confidence interval (CI) [2.74; 20.90]; goodness of fit: Chi-square=17.97233; p<0.0001.
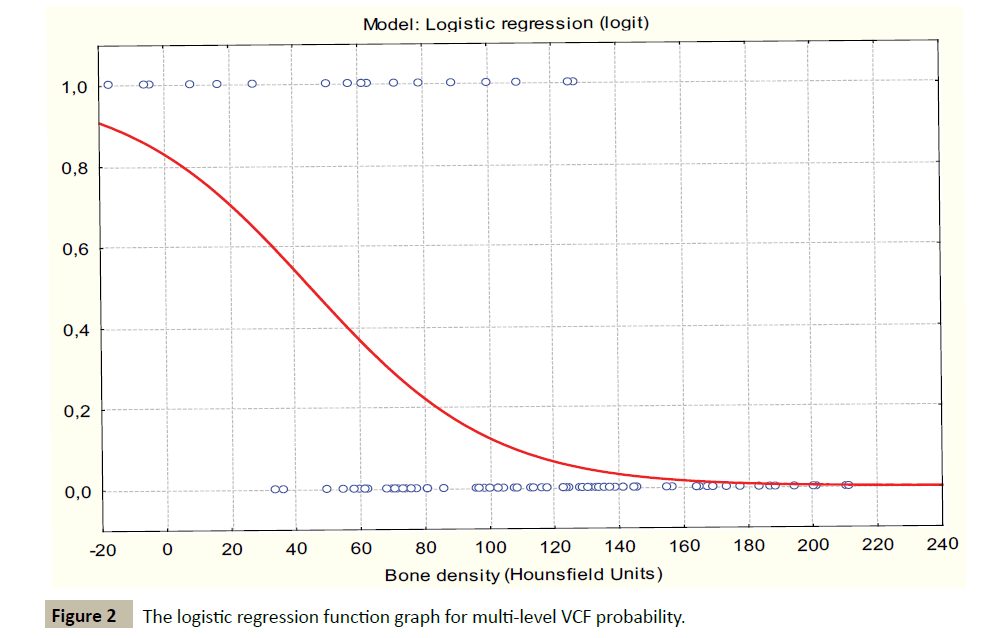
The incidence of multi-level VCF was found to have a strong inversely relationship to bone density. The parameters of the logistic regression models were: ÃÆÃÂÃâââ¬â¢0=1.5934, p=-0.0338; B1=0.0355, p=0.00013. Goodness of fit: Chi-square=27.21870; p<0.0001, percent of correctly predicted 86% (Figure 2).
The logistic regression model demonstrated a critical inflection point of 82.0 HU associated with a significant increase in rate of multi-level VCF (critical point associated with multilevel VCF probability growth acceleration was detected using derivatives of logistic regression function). The estimated logistic regression for this value was B0=0.5261 p=0.1358 (not significant); B1=1.9588, p=0.000, 0.011; Odds ratio(OR)=7.09; 95% confidence interval (CI) [2.23; 22.51]; goodness of fit: Chisquare= 12.84427; p=0.0003.
Degenerative changes were found to correlate with a decreased likelihood of VCF. The parameters of the logistic regression model were Chi-square=8.976428 p=0.0027; ÃÆÃÂÃâââ¬â¢0=0.3514, ÃÆââ¬ËÃâââ¬=0.2434 (not significant); B1=1.3978, p=0.0050 OR=4.04 [1.54;10.62]. Table 3 shows the breakdown of degenerative changes seen in this study.
| Patients | Fractures | |
|---|---|---|
| Subgroup without degenerative changes | 46 | 19 (41%) |
| Subgroup with degenerative changes | 54 | 8 (15%) |
Table 3: Classification of cases based on presence of degenerative changes.
Discussion
Although there are many reports demonstrating the correlation between reduced bone mineral density as measured by DEXA and low energy VCF [21-25], no clear cutoff for fracture risk has been identified. Furthermore, changes in bone mineral density as measured by DEXA have not always had a strong correlation to changes in fracture risk assessment [26]. Bone mineral density as measured by DEXA has a low predictive value for predicting the risk of future fractures [27,28]. For these reasons, some have recommended that DEXA values be combined with other clinical factures or diagnostic studies when estimating the risk of VCF [29-33]. In distinction, the detection of even a minimal VCF, is highly predictive of future fracture risk [33-36]. It would however but more clinically useful to establish the risk of a fracture before it occurs so that prophylactic treatment could be instituted to prevent fracture [37].
One explanation for the observed discrepancy in the predictive value of DEXA is the fact that DEXA provides an areal estimate of the integral BMD of the entire vertebra including both cortical and trabecular bony structures [17-38]. Additionally, the values provided by DEXA can be highly affected by bone size, spinal deformity or the presence of degenerative changes [17- 39]. In distinction, CT provides a true volumetric bone density independent of bone size and allows the trabecular bone to be sampled independent from the endplates and posterior elements [18-44]. In also allows the examiner to identify the presence of degenerative changes which have a prognostic impact on fracture risk as shown in the present analysis. For these reasons, quantitative CT has clear advantages in terms of predicting low energy fracture risk compared to DEXA [17,18].
The present study confirms that CT measurements have a strong relationship with VCF risk. A clinically useful finding of this study is significant correlation with increased VCF risk for patients with a bone density of less than 101 HU. Additionally, the risk of multilevel VCF is significantly increased when the bone density falls below 82 HU. Additionally, this study has confirmed what could be clinically suspected that patient with degenerative changes are less likely to suffer a VCF. This effect is likely due to the increased mechanical strength of the sclerotic endplates which stress shield the vertebral body in erect posture [45].
Although highly predictive, routine use of CT as a screening tool for osteoporosis is unsuitable due to the associated radiation exposure and cost of the study compared to DEXA. However, when CT scans are obtained for other clinical indications, they afford the clinician a unique opportunity to easily obtain useful data that can be used to direct treatment and counsel patients with regards to fracture risk. In this capacity, CT is provides excellent insights into bone density and an opportunity to make prognostic inferences is obtained.
Thus study has certain limitations which must be acknowledged. Only patients with axial pain requiring CT scanning for workup were included in this study. It is plausible that the asymptomatic population would have a different distribution of findings. In addition, the number of participants in this study is relatively small, however the power analysis confirmed that the sample size was sufficient to support the conclusions reached.
Conclusion
In conclusion, the results of our study show that bone density measured by CT can be used to predict the risk of low energy VCF. Bone density above 150 HU is highly unlikely to be associated with low energy VCF. In contrast, bone density of 101 HU or less is associated with an increased risk of VCF, and bone density of less than 82 HU is associated with an increased risk of multi-level VCF. Clinicians can recommend preventive therapies and provide counseling for high risk cases to reduce the chances of future VCF.
References
- Johnell O, Kanis J (2005) Epidemiology of osteoporotic fractures. Osteoporosos Int 16: S3-7.
- Felsenberg D, Silman AJ, Lunt M, Armbrecht G, Ismail AA, et al. (2002) Incidence of vertebral fracture in Europe: results from the European Prospective Osteoporosis Study (EPOS). J Bone Miner Res 17: 716-724.
- Melton LJ, Kan SH, Frye MA, Wahner HW, O’Fallon WM (1989) Epidemiology of vertebral fractures in women. Am J Epidemiol 129: 1000-1011.
- Hodsman AB, Leslie WD, Tsang JF, Gamble GD (2008) 10-year probability of recurrent fractures following wrist and other osteoporotic fractures in a large clinical cohort: an analysis from the Manitoba Bone Density Program. Arch Intern Med 168: 2261-2267.
- Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, et al. (1996) Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 81: 4358-4365.
- LaFleur J, McAdam-Marx C, Kirkness C, Brixner DI (2008) Clinical risk factors for fracture in postmenopausal osteoporotic women: a review of the recent literature. Ann Pharmacolther 42: 375-386.
- McLeod KM, Johnson CS (2009) Identifying women with low bone mass: a systematic review of screening tools. Geriatr Nurse 30: 164-173.
- Cortet B, Roches E, Logier R, Houvenagel E, Gaydier-Souquières G, et al. (2002) Evaluation of spinal curvatures after a recent osteoporotic vertebral fracture. Joint Bone Spine 69: 201-208.
- Pluijm SM, Tromp AM, Smit JH, Deeg DJ, Lips P (2000) Consequences of vertebral deformities in older men and women. J Bone Miner Res 15: 1564-1572.
- Schlaich C, Minne HW, Bruckner T, Wagner G, Gebest HJ (1998) Reduced pulmonary function in patients with spinal osteoporotic fractures. OsteoporosInt 8: 261-267.
- Yi Y, Hwang B, Son H, Cheong I (2012) Low bone mineral density, but not epidural steroid injection, is associated with fracture in fostmenopausal women with low back pain. Pain Physician 15: 441-449.
- Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19: 385-397.
- Black DM, Steinbuch M, Palermo L, Dargent-Molina P, Lindsay R, et al. (2001) An assessment tool for predicting fracture risk in postmenopausal women.OsteoporosInt 12: 519-528.
- Mull RT (1984) Mass estimates by computed tomography: physical density from CT numbers. AJR Am J Roentgenol 143: 1101-1104.
- Merheb J, Van Assche N, Coucke W, Jacobs R, Naert I, et al. (2010) Relationship between cortical bone thickness or computerized tomography-derived bone density values and implant stability. Clin Oral Implants Res 21: 612-617.
- Hiasa K, Abe Y, Okazaki Y, Nogami K, Mizumachi W, et al. Preoperative computed tomography-derived bone densities in hounsfield units at implant sites acquired primary stability. ISRN Dent 2011;2011: 678729.
- Rehman Q, Lang T, Modin G, Lane NE (2002) Quantitative computed tomography of the lumbar spine, not dual X-Ray absorptiometry, is an independent predictor of prevalent vertebral fractures in postmenopausal women with osteopenia receiving long-term glucocorticoid and hormone-replacement therapy. Arthritis Rheum 46: 1292-1297.
- Naganathan V, Jones G, Nash P, Nicholson G, Eisman J, et al. (2000) Vertebral fracture risk with long-term corticosteroid therapy: prevalence and relation to age, bone density, and corticosteroid use. Arch Intern Med 160: 2917-2922.
- Pickhardt PJ, Lee LJ, del Rio AM, Lauder T, Bruce RJ (2011) Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res 26: 2194-2203.
- Genant HK, Wu CY, Vankuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. Journal of Bone and Mineral Research 8: 1137-1148.
- Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Brit Med J 312: 1254-1259.
- Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, et al. (1995) Risk factors for hip fracture in white women. N Engl J Med 332: 767-773.
- Bouxsein ML, Palermo L, Yeung C, Black DM (2002) Digital X-ray radiogrammetry predicts hip, wrist and vertebral fracture risks in elderly women. A prospective analysis from the study of osteoporotic fractures. OsteoporosInt 13: 358-365.
- Kanis JA, Melton III LJ, Christiansen C, Johnston CC Jr, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9: 1137-1141.
- Lewis CE, Ewing SK, Taylor BC, Shikany JM, Fink HA, et al. (2007) Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res 22: 211-219.
- Small RE (2005) Uses and Limitations of Bone Mineral Density Measurements in the Management of Osteoporosis. Med Gen Med 7: 3.
- Freitas SS, Barrett-Connor E, Ensrud KE (2008) Rate and circumstances of clinical vertebral fractures in older men. OsteoporosInt 19: 615-623.
- Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, et al. (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34: 195-202.
- Jager PL, Jonkman S, Koolhaas W, Stiekema A, Wolffenbuttel BH, et al. (2011) (Combined vertebral fracture assessment and bone mineral density measurement: a new standard in the diagnosis of osteoporosis in academic populations. OsteoporosInt 22: 1059-1068.
- Vokes TJ, Gillen DL (2010) Using clinical risk factors and bone mineral density to determine who among patients undergoing bone densitometry should have vertebral fracture assessment. OsteoporosInt 21: 2083-2091.
- Cepollaro C, Gonnelli S, Pondrelli C, Martini S, Montagnani A, et al. (1997) The combined use of ultrasound and densitometry in the prediction of vertebral fracture. Br J Radiol 70: 691-696.
- Lewiecki EM, Laster AJ (2006) Clinical review: clinical applications of vertebral fracture assessment by dual-energy X-ray absorptiometry. J Clin Endocrinol Metab 91: 4215-4222.
- Xu W, Perera S, Medich D, Fiorito G, Wagner J (2011) Vertebral Fractures, and the Misclassification of Osteoporosis. Bone 48: 307-311.
- Melton LJ III, Atkinson EJ, Cooper C, O’Fallon WM, Riggs BL (1999) Vertebral fractures predict subsequent fractures. OsteoporosInt 10: 214-221.
- Cauley JA, Hochberg MC, Lui LY, Palermo L, Ensrud KE, et al. (2007) Long-term risk of incident vertebral fractures. JAMA 298: 2761-2767.
- Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA III, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15: 721-739.
- Ferrar L, Jiang G, Adams J, Eastell R (2005) Identification of vertebral fractures: an update. Osteoporos Int 167: 717-728.
- Blake GM, Fogelman I (1997) Technical principles of dual energy x-ray absorptiometry. Semin Nucl Med 27: 210-228.
- Tenne M, McGuigan F, Besjakov J, Gerdhem P, Åkesson K (2013) Degenerative changes at the lumbar spine-implications for bone mineral density measurement in elderly women. OsteoporosInt 24: 1419-1428.
- Leslie WD, Tsang JF, Caetano PA, Lix LM (2007) Manitoba bone density program. Effectiveness of bone density measurement for predicting osteoporotic fractures in clinical practice. J Clin Endocrinol Metab 92: 77-81.
- Genant HK, Engelke K, Fuerst T, Glüer CC, Grampp S (1996) Noninvasive assessment of bone mineral and structure: state of the art. J Bone Miner Res 11: 707-730.
- Grampp S, Jergas M, Glüer CC, Lang P, Brastow P, et al. (1993) Radiologic diagnosis of osteoporosis: current methods and perspectives. Radiol Clin North Am 31: 1133-1145.
- Liu G, Peacock M, Eilam O, Dorulla G, Braunstein E, et al. (1997) Effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. OsteoporosInt 7: 564-569.
- Grampp S, Genant HK, Mathur A, Lang P, Jergas M (1997) Comparisons of noninvasive bone mineral measurements assessing age-related loss, fracture discrimination, and diagnostic classification. J Bone Miner Res 12: 697-711.
- Pollintine P, Dolan P, Tobias JH, Adams MA (2004) Intervertebral disc degeneration can lead to "stress-shielding" of the anterior vertebral body: a cause of osteoporotic vertebral fracture? Spine 29: 774-782.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences