Nanomaterials for Spinal Cord Injury Recovery
Rodolfo Amezcua, Gina Vimbela and David A Stout
DOI10.21767/2471-8173.100026
1Department of Mechanical and Aerospace Engineering, California State University, Long Beach, CA, USA
2Department of Chemical Engineering, California State University, Long Beach, CA, USA
3Department of Biomedical Engineering, California State University, Long Beach, CA, USA
4International Research Center for Translational Orthopaedics, Soochow University, Suzhou, China
- *Corresponding Author:
- David A Stout
Department of Mechanical and Aerospace Engineering, Department of Biomedical Engineering, International Research Center for Translational Orthopaedics, California State University, Long Beach, CA 90840, USA
Tel: +15629851502
Email: david.stout@csulb.edu
Received date: Oct 25, 2016; Accepted date: Mar 17, 2017; Published date: Mar 22, 2017
Citation: Stout DA, Vimbela G, Stout DA. Nanomaterials for Spinal Cord Injury Recovery. Spine Res 2017, 3:1. doi:10.21767/2471-8173.10003
Abstract
Nanotechnology and nanomaterials have had a significant positive impact within the biomedical field for quite some time, and have included cardiovascular, cartilage, and neural tissue engineering applications. Due to its potential for treating neural tissue, current research is investigating the use of nanomaterials for spinal cord injury (SCI), an injury characterized by tissue damage and the disruption of communication between the brain to the body. To treat such an injury, cell-based therapy has shown promising results, and the following papers are recommended. This communication will focus on nanoparticle, carbon nanotubes, and self-assembling peptide approaches for treating SCI, as well as concerns of toxicity.
Keywords
Nanotechnology; Cardiovascular; Density; Cytokines
Abbreviations
CBN: Carbon Based Nanomaterial; IKVAV: Isoleucine-Lysine- Valine-Alanine-Valine; MWCNT: Multi Walled Carbon Nanotubes; PA: Peptide Amphiphile; SCI: Spinal Cord Injury; SPIO: Superparamagnetic Iron-Oxide; SWCNT: Single Walled Carbon Nanotubes; GLAST: GLutamate Aspartate Transporter; PEG: Polyethylene Glycol; PEGDA: {DD{AEP (Polyethylene Glycol Diacrylate{Dodecylamine{1-(2-Aminoethyl)Piperazine); PNIPAAM: Poly(n-Isopropylacrylamide); PEDOT: Poly(3,4- Ethylene Dioxythiophene)
Introduction
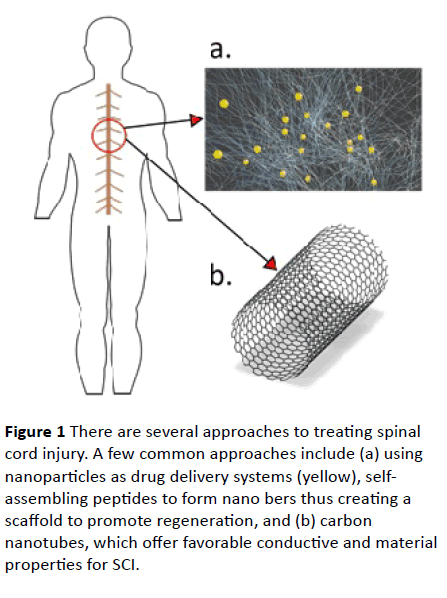
Nanotechnology and nanomaterials have had a significant positive impact within the biomedical field for quite some time, and have included cardiovascular, cartilage, and neural tissue engineering applications [1,2]. Due to its potential for treating neural tissue, current research is investigating the use of nanomaterials for spinal cord injury (SCI), an injury characterized by tissue damage and the disruption of communication between the brain and the body. To treat such an injury, cell-based therapy has shown promising results, and the following papers are recommended [3,4]. This communication will focus on nanoparticle, carbon nanotubes, and self-assembling peptide approaches for treating SCI (Figure 1), and will also address toxicity concerns.
Figure 1 There are several approaches to treating spinal cord injury. A few common approaches include (a) using nanoparticles as drug delivery systems (yellow), selfassembling peptides to form nano bers thus creating a scaffold to promote regeneration, and (b) carbon nanotubes, which offer favorable conductive and material properties for SCI.
Nanoparticle Approaches
Nanoparticles are popularly used as a drug delivery system, because they are capable of crossing the cell membrane due to their size. Wu et al. have shown that by using nanoparticles composed of ferulic acid modified glycol chitosan, there is an improved circulation time of the particles, and can arrive at both gray and white matter [5]. Their spinal cord contusion injury rat model exhibited significant locomotor function recovery after intravenously receiving nanoparticles two hours after the injury. The significance of this study lies in their success of observing therapeutic effects after a prolonged post-treatment time period.
Drug-loaded poly (methyl methacrylate) nanoparticles show potential for administrating treatment in activated microglia and macrophages to reduce secondary in ammatory events in SCI [6]. Papa et al. found that these nanoparticles exhibited internalization within microglial cells after thirty minutes and plateaued after three hours of treatment. Furthermore, by tuning the surface charge and PEGylation, the cell uptake can be controlled.
Another study proposes using methylprednisolone-loaded dendrimer nanoparticles to produce a favorable microglial post-injury response and viability [7]. The left dorsal side of their rat models' spinal cord was surgically removed, thus impairing locomotion. After administration of the nanoparticles, the models show significant improvement in their functional outcome; the authors attribute this result to the sustained release of methylprednisolone modulating the in ammation following injury, specifically the microglial population density.
To efficiently repair damage to the spinal cord, a group suggests introducing gold nanoparticles to 3D nano ber scaffolds [8]. Gold nanoparticles were loaded onto the surface of electrospun PCL/gelatin nano bers. Neuronal cells were seeded onto the nanocomposite scaffolds, resulting in the growth of elongated axons forming 3D networks.
Furthermore, because superparamagnetic iron-oxide (SPIO) nanoparticles can be visualized by MRI, this material has successfully been used for in vivo tracking of transplanted cells. This technology offers a noninvasive method to determine successful cell engraftment, and monitor cell migration and viability [9].
Self-Assembling Peptide Approaches
Self-assembling peptides o er a convenient way to combat SCI because they o er a non-invasive procedure by forming nano fibers upon injection into tissue. For example, one group succeeded in using peptide amphiphile (PA) molecules enhanced with isoleucine-lysine-valine-alanine-valine (IKVAV) that self-assemble into nano fibers for mice SCI therapy [10]. IKVAV is found in laminin and is known to promote neurite sprouting and to direct neurite growth [11]. After nine weeks from the SCI injury, mice within the IKVAV PA group showed significant improvement. They attribute the favorable results to the fact that the nano fibrils inhibited scar formation.
IKVAV PA consistently shows promising contributions to developing SCI therapy. Tysseling et. al used this injectable self-assembling peptide with both their mice and rat models. This group suggests the increased serotonergic bers in the caudal spinal cord and regeneration of the dorsal column sensory axons were due to the IKVAV PA injection. It also produced functional improvements within their specimens [12]. To compare the results after injection between IKVAV PA and control groups, the study used the BBB scale for modified mouse [13].
RADA16-I peptide, a type of self-assembling nano ber scaffold, has also been shown to promote neural progenitor cell and Schwann cell survival, migration, and differentiation both in vitro and in vivo [14]. When the scaffold is pretreated with culture medium before transplantation, the implants integrate well and show a significant number of host cells that migrate into the scaffold. This two-way cell migration between the scaffold and host tissue is essential behavior towards efficient SCI repair.
Liu et al. used peptide QL6, which self-assembles into-sheets at neutral pH, as their therapeutic agent for SCI [15]. This study found a favorable distribution of the nano bers after injection and almost full degradation by eight weeks. Furthermore, their scaffold exhibited reduced astrogliosis and in ammation. The group suggests that QL6 allows for axonal preservation and regeneration due to its capability of inhibiting glial scar formation and reducing in ammation.
Carbon Nanotube Approaches
Carbon nanotubes are cylindrical structures with a radius within the 1 nm to 100 nm range, composed of a concentric geometry of a single cylinder (single-walled) or multiple cylinders (multi-walled) [16]. These carbon-based nanostructures feature conductive and mechanical properties favorable for neurological applications, in addition to showing a desirable impact on neuronal cell morphology and excitability.
The neurotransmitter glutamate has been demonstrated to lead to neuronal cell death during excitotoxic processes. To maximize the uptake of extracellular glutamate by astrocytes after spinal cord injury, a study developed polyethylene glycol functionalized single-walled carbon nanotubes (SWCNT-PEG) [17]. The functionalized carbon nanotubes were introduced to cortical astrocytes from neonatal mouse pups in solution. Images of the astrocytes showed favorable effects on the morphology of the astrocytes in addition to an increase in the reactivity of the glutamate transporter GLAST on the cell surface. The authors suggest the results could minimize the progressive degenerative effects that occur after a spinal cord injury.
Sang et al. co-polymerized PEGDA-DD-AEP, n-isopropyl acrylamide, and SWCNTs to produce a heat sensitive injectable hydrogel for promoting nerve tissue regeneration [18]. After the SWCNT {PNIPAAM hydrogel was injected into a SCI rat model, images showed neuronal migration into the injury site. Results presented a reduction of nerve tissue scarring after hydrogel implantation.
Another possible treatment for SCI is providing stimulation to the dorsal root ganglion. By combining the conducting polymer PEDOT and MWCNTs, Kolarcik et al. developed a coating material for the electrode surface to decrease impedance at electrode-tissue interface [19]. The coating material was also doped with dexamethasone, an anti-in ammatory drug. The addition of the electrode coating resulted in a significant decrease in neuronal cell damage and death while doping with dexamethasone caused a reduced in ammatory response.
Toxicity Concerns
The clinical relevance of SCI treatment that exploits the enhanced material properties of nanotechnology depends heavily on whether researchers can confirm non-toxic effects on the patient. This topic has been heavily debated for quite some time now, especially because of the multitude of variables at hand when using nanostructures in a biological system. For example, there exists significant variability in the manufacturing methods, raw materials, and reaction scaling to create uniform nanomaterials [20]. Furthermore, several material properties affect their interaction with the biological system such as size, shape, surface area, chemical composition, lattice structure, surface chemistry, surface charge, and aggregation state [21].
Carbon-based nanomaterials (CBNs) are highly controversial. There have been reports of adverse effects through inhalation of CBNs, cell death, and inhibited cell proliferation [22,23]. To circumvent this issue, functionalization of CBNs is a standard approach that directly affects the degree of cytotoxicity [24]. Nonetheless, studies using CBNs for SCI should specifically address toxicity, and go as far as determining a dosage threshold. Thus, the effects of CBNs in biological systems must be adequately quantified before offering any practical clinical treatment.
Outlook on Future Research
Significant progress for developing SCI treatment has been made; however, there are still several obstacles to overcome. For example, often proposed therapies require an almost immediate application (fifteen minutes), thus severely limiting its practicality. Scaffolds' degradation and release rates of implanted growth factors or cytokines may also pose an issue. These scaffolds must promote an adequate response from seeded materials, such as cells or drugs, but must also allow for host cells to thrive all while keeping in ammation to a minimum. Furthermore, as researchers turn to more natural scaffolds, the material properties of the gels become increasingly complex. It is crucial to further understand the effects of these variables on both the host cells and the neighboring tissue.
Carbon-based nanomaterials are appealing for treatment of neuronal tissue because of their unique conductive properties. Additionally, new approaches for modifying the surface of CNTs, either by functionalization or drug loading, suggest that the full extent of these nanostructures' application is still largely unknown. However, the toxicity of CNTs remains a concern. In order to someday use carbon-based nanomaterials in a clinical setting, it is critical to gain a greater understanding of the toxic effects of CNTs on specific tissue types.
References
- Zhang L, Webster T (2001) Nanotechnology and nanomaterials: Promises for improved tissue regener-ation. Nano Today 4: 66-80.
- Amezcua R, Shirolkar A, Fraze C, Stout D (2016) Nanomaterials for cardiac myocyte tissue engineering. Nanomaterials 6: 133.
- Nakamura M, Okano H (2013) Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell res 23 :70-80.
- Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukain M, et al. (2010) Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci USA 107: 12704-12709.
- Wu W, Lee S, Wu X, Tyler J, Wang H, et al. (2014) Neuroprotective ferulic acid (fa){glycol chitosan (gc) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials 35: 2355-2364.
- Papa S, Ferrari R, Paola M, Rossi F, Mariani A, et al. (2014)Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J Control Release 174: 15-26.
- Cerqueira S, Oliveira J, Silva N, Leite-Almeida H, Ribeiro-Samy S, et al. (2013) Microglia response and in vivo thera-peutic potential of methylprednisolone-loaded dendrimer nanoparticles in spinal cord injury. Small 9: 738-749.
- Baranes K, Shevach M, Tal O (2016) Gold nanoparticle-decorated scaffolds promote neuronal di erentiation and maturation. Nano Lett 16: 2916-2920.
- Kubinova S, Sykova E (2010) Nanotechnology for treatment of stroke and spinal cord injury. Nanomedicine 5: 99-108.
- Tysseling-Mattiace V, Sahni V, Niece K, Birch D, Czeisler C (2008)Self-assembling nano bers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci 28: 3814-3823.
- Silva G, Czeisler C, Niece K, Beniash E, Harrington D (2004) Selective di erentiation of neural progenitor cells by high-epitope density nano bers. Science 303: 1352-1355.
- Tysseling V, Sahni V, Pashuck E, Birch D, Hebert A, et al. (2010) Self-assembling peptide amphiphile promotes plasticity of serotonergic bers follow-ing spinal cord injury. J neurosci res 88: 3161-3170.
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, et al. (1996) Mascis evaluation of open field locomotor scores: Effects of experience and teamwork on reliability. J neurotrauma 13(7): 343-359.
- Guo J, Su H, Zeng Y, Liang Y, Wong W, et al. (2007) Reknitting the injured spinal cord by self-assembling peptide nano ber scaffold. Nanomedicine 3: 311-321.
- Liu Y, Ye H, Satkunendrarajah K, Yao G, Yves Bayon, et al. (2013) A self-assembling peptide reduces glial scarring, attenuates post-traumatic in ammation and promotes neurological recovery following spinal cord injury. Acta biomaterialia 9: 8075-8088.
- Balasubramanian K, Burghard M (2005) Chemically functionalized carbon nanotubes. Small 1: 180-192.
- Gottipati MK, Bekyarova E, Haddon R, Parpura V (2005) Chemically functionalized single-walled carbon nanotubes enhance the glutamate uptake characteristics of mouse cortical astrocytes. AMINO ACIDS 47: 1379-1388.
- Sang L, Liu Y, Hua W, Xu K, Wang G, et al. (2016) Thermally sensitive conductive hydrogel using amphiphilic cross linker self-assembled carbon nanotube to enhance neurite outgrowth and promote spinal cord regeneration. RSC Adv 6: 26341-26351.
- Kolarcik CL, Catt K, Rost E, Albrecht I, Bourbeau D, et al. (2015) Evaluation of poly(3,4-ethylenedioxythiophene)/carbon nanotube neural electrode coatings for stimulation in the dorsal root ganglion. J Neural Eng 12: 016008.
- Fischer HC, Chan WCW (2007) Nanotoxicity: The growing need for in vivo study. Cur opinion biotechnol 18: 565-571.
- Webster T, Stout D, Kalmodia S, Basu B (2013) Experimental methods and in vitro cytoxicity and genotoxicity of nanomaterials. Nano LIFE 3: 1340008.
- Magrez A, Kasas S, Salicio V, Pasquier N, Seo W, et al. (2006) Cellular toxicity of carbon-based nanomaterials. Nano lett 6: 1121-1125.
- Buzea C, Pacheco I, Robbie K (2007) Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2: MR17-MR71.
- Sayes C, Liang F, Hudson J, Mendez J, Guo W, et al. (2006) Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol lett 161: 135-142.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences