A Global Pictorial Assessment of the Intervertebral Disc Cell in Several Species Reveals a Remarkable Biodiversity in this Cell Type which should be taken into Account in Experimental Studies on Intervertebral Disc Repair
Melrose J
DOI10.21767/2471-8173.100013
1Raymond Purves Bone and Joint Research Laboratory, Kolling Institute Northern Sydney Local Health District, St. Leonards, NSW 2065, Australia
2Sydney Medical School, Northern, The University of Sydney, Royal North Shore Hospital, Australia
3School of Biomedical Engineering, University of New South Wales, Kensington, NSW 2052, Australia
- *Corresponding Author:
- Melrose J
Raymond Purves Laboratory, Institute of Bone and Joint Research, Kolling Institute of Medical Research, Level 10, Kolling Building B6, The Royal North Shore Hospital St Leonards, NSW 2065, Australia.
Tel: 61299264806
Fax: 61299265266
E-mail: james.melrose@sydney.edu.au
Received date: March 26, 2016; Accepted date: April 25, 2016; Published date: April 28, 2016
Citation: Melrose J. A Global Pictorial Assessment of the Intervertebral Disc Cell in Several Species Reveals a Remarkable Biodiversity in this Cell Type which should be taken into Account in Experimental Studies on Intervertebral Disc Repair. SpineRes. 2016, 2:1. doi:10.21767/2471-8173.100013
Abstract
The intervertebral disc (IVD) is an intricate collection of connective tissues of disparate structure and function, which collectively act as a weight-bearing cushion between the bony vertebrae of the spine. The outer region of the IVD is the annulus fibrosus (AF), a collagen-rich tissue consisting of successive layers or lamellae which contain the hoop stresses generated in the IVD upon axial compression of the spine, these provide the AF with tensile properties which aid in the dissipation of these compressive loads . The AF encloses the central region of the IVD, the nucleus pulposus (NP) which is a gelatinous tissue (at least in infancy) rich in proteoglycans (aggrecan) that imbibe water and provide hydrodynamic weight bearing properties to the composite IVD.
Keywords
Intervertebral disc; Disc progenitor cells; Disc cell morphology; Notochordal cell
Introduction
The intervertebral disc (IVD) is an intricate collection of connective tissues of disparate structure and function, which collectively act as a weight-bearing cushion between the bony vertebrae of the spine [1]. The outer region of the IVD is the annulus fibrosus (AF), a collagen-rich tissue consisting of successive layers or lamellae which contain the hoop stresses generated in the IVD upon axial compression of the spine, these provide the AF with tensile properties which aid in the dissipation of these compressive loads [2]. The AF encloses the central region of the IVD, the nucleus pulposus (NP) which is a gelatinous tissue (at least in infancy) rich in proteoglycans (aggrecan) that imbibe water and provide hydrodynamic weight bearing properties to the composite IVD. Superior and inferior layers of hyaline-like cartilage, the cartilaginous endplates (CEP), further contain the NP cranially and caudally interfacing between the IVD and adjacent vertebral bodies (VBs) preventing the extrusion of the NP into the VBs under the very considerable compressive loads it experiences during axial spinal loading [3]. The IVD tissue components are synthesised, assembled and maintained by a number of different cell types. The AF contains an elongated fusiform fibroblast-like collection of interconnected cells which are layed down between and within the fibrillar collagenous lamellar layers. In the inner AF these cells assume a plumper more rounded morphology than the outer AF cells. The cells of the NP are reported to be large and vacuolated in the newborn but soon after birth they become smaller and occur as single rounded chondrocytelike cells surrounded by a well-defined pericellular matrix set within an abundant interstitial and interterritorial matrix rich in aggrecan and type II collagen. The cells of the CEP have rounded chondrocyte-like morphologies and are surrounded by a glassy hyaline cartilaginous matrix which merges with the fibrocartilaginous NP and AF and the bone of the vertebral body.
Ontogeny of IVD cells
During embryogenesis the notochord is surrounded by mesenchymal cells which have important roles to play in the development of the AF, NP and VB. The earliest stages of vertebral development are identified in the primitive streak germ layers and notochord during the first 3 weeks of gestation. Cellular migration occurs from the primitive streak to form the somites, dermatomyotome and sclerotome [4]. Cells subsequently migrate from the sclerotome to surround the notochord and areas of high and low sclerotomal cell density subsequently develop by weeks 5-6, these will give rise to the IVD and VBs [5]. Condensed notochordal cells centrally within the developing AF subsequently give rise to the NP over weeks 7-9. Progressive maturation of the annular lamellae and NP occurs over weeks 9-12 of gestation along with a gradual regression of the notochord with chondrogenesis driving early spinal development. Hypertrophy of central chondrocytes in the VB rudiment cartilages initiate formation of the primary ossification centres which begin to mineralise from week 10 of gestation. The IVD undergoes progressive maturational changes over foetal weeks 10-20 better defining the AF and NP, increases in IVD glycosaminoglycan content also occur and the development of the characteristic collagenous annular arcades inserting into the VBs.
Characteristics of notochordal cells
Notochordal cells are the progenitor cell type for the small rounded fibrochondrocytic NP cells (~10 µm diameter) which populate the adult IVD. Foetal notochordal cells are large (25- 85 µm) and contain intracellular vacuole-like structures leading to their description as a physalipherous (foamy) cell type, with an amorphous nucleus, well demarcated Golgi and extended endoplasmic reticulum [6]. At birth, ECM deposition within the NP notochordal remnant separates the compact foetal notochordal cells into looser cell clusters (chorda reticulum). In man it is suggested that these foetal notochordal cells disappear by 11 years of age however there are also reports of a small percentage of notochordal cells which persist in the adult IVD. This change from large notochordal cell to small NP cell in the adult IVD occurs in humans, bovine, horse and chondrodystrophic dogs however pigs, rabbits, non-chondrodystrophic dogs, mice and rats retain the large vacuolated NP cell phenotype till much later in life and some strains of these animals even retain the large vacuolated notochordal cell type into old age [7]. Fate mapping cell lineage studies using tamoxifen inducible ShhCreERT2 and NotoCre mice have shown unequivocally that all NP cells including those in the adult have a notochordal origin [8]. Large and small cells in the adult NP have also been shown to express Brachyury, a notochord specific protein. Rats, chondrodystrophoid dogs, adult bovine, degenerate and non-degenerate human NP cells all express Brachyury in adulthood [9]. Furthermore, analysis of morphologically different NP cells ie small chondrocyte-like and large vacuolated cells showed a significant overlap in gene expression profiles including Brachyury which suggests a common ancestry. The expression of Brachyury by all NP cells also shows that adult NP cells with morphologies dissimilar from what are considered notochordal cells also exist. These may represent adult "notochordal cells" which have undergone maturational change resulting in a different morphology from the cells residing in the foetal notochord [10,11]. Isolation and culture of single cells and cell clusters from degenerate human IVDs has shown that the clustered cells proliferate more slowly in monolayer culture [12]. Both cell populations expressed notochordal and progenitor cell markers including the CS sulphation motifs 3B3(-), 7D4, 4C3 and 6C3; Notch-1, and cytokeratins -8 and -19. Flow cytometry also demonstrated stem cell marker (CD73, CD90, CD105) profiles similar to those displayed by bone marrow derived stem cells.
In an alternative approach, time-lapsed cell tracking methodology on single notochordal cells and a robotic automated real-time cell imaging system has shown that some notochordal cells grown in 3D culture eventually displayed a chondrocyte-like phenotype synthesising type II collagen and aggrecan, these notochordal cells therefore represented a progenitor cell population responsible for the formation of the NP cells [13], other notochordal cells formed giant cells in culture but did not divide.
The adult notochordal cell controversy
Most adult mammals have IVDs containing fibroblastic and chondrocytic cells but few notochordal cells, or at least cells of a physalipherous morphology [14]. Some authors have however reported the persistence of the notochord in the adult human spine and the presence of IVD cells which express typical notochordal markers, these cells however are not of the same morphology as the foetal notochordal cells. Their identity in the adult IVD is based on their relatively large size, occurrence in cell clusters in the NP and the expression of well-defined notochordal markers such as Brachyury. Adult notochordal cells also express cytokeratin-8 and -19 and a number of progenitor cell markers (Notch-1, 3B3[-], 4C3, 7D4, CD73, CD90, CD105) which are less specific [12]. A major problem is that a clear description of the definitive morphological characteristics of an adult notochordal cell does not exist in the literature. This leaves authors to make the assumption that cells of a different morphology to that of the small fibrochondrocytic NP cells must therefore represent an adult notochordal cell population and a number of researchers have now shown that these cells express notochordal markers. We consider that calling these cells notochordal is not helpful and that they should be considered as an adult progenitor cell population derived from a notochordal cell population which has undergone maturational change into this progenitor cell population. In the present study we have identified such a population of cells of very similar morphology to NP cells recently identified in human IVDs and which expressed notochordal cell markers and displayed CD marker profiles similar to stem/ progenitor cells [12]. In future work we aim to fully characterise this cell type and determine its suitability for IVD repair.
Materials and Methods
Chondroitinase-ABC was obtained from Sigma-Aldrich, Castle Hill, NSW, Australia. Keratanase I and Keratanase II were obtained from Seikegaku Japan. Menzel and Glaser SuperFrost ultraPlus, positively charged microscope sides were obtained from Fisher Scientific, Braunschweig, GmbH. Biotinylated antimouse, IgG secondary antibody, and streptavidin horseradish peroxidase conjugate were obtained from Dako, Botany, NSW, Australia. Histochoice® was an Amresco product (Solon, OH, USA). Monoclonal antibody (mAb) to aggrecan G1 domain (clone 969D4D11) was obtained from Biosource Europe, Nivelle, Belgium through Bioclone, Sydney, Australia. MAb 12C5 to versican G1 domain originally developed by Dr R Asher [15,16] was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. NovaRED substrate was obtained from Vector laboratories, Burlingame, CA, USA.
Tissues
Histological processing of IVDs
Prior to fixing the spinal segments or individual IVDs, the specimens were trimmed of all surrounding soft connective tissue and subjected to "En-bloc" fixation for 48h in 10% neutral buffered formalin or Histochoice (human foetal and mouse specimens). The specimens were then decalcified in 10% (v/v) formic acid 5% (v/v) neutral buffered formalin with constant agitation and frequent changes of decalcification solution for up to 10 days. The decalcifications were carefully monitored to an end point where a pin could be inserted into the vertebral bodies with modest pressure. Entire mouse and human foetal spinal segments, individual fallow deer or sheep newborn IVDs or vertical tissue blocks (~5 mm thick) of fixed and decalcified IVDvertebral body segments were dehydrated in graded ethanols and xylene and embedded in paraffin wax. Four micron vertical microtome sections were cut from the fixed decalcified tissue blocks. These were attached to SuperFrost Plus glass microscope slides (Menzel-Glaser, Germany), de-paraffinised in xylene (2 changes × 5 min), and re-hydrated through graded ethanol washes (100-70% v/v) to water.
Histochemistry
Toluidine blue staining
Mid-line longitudinal sections of the mouse and human foetal spinal segments (4 m) were prepared. Vertical sections (4 m) of IVD and superior and inferior vertebral bodies of the fallow deer and sheep specimens were stained for 10 min with 0.04% (w/v) toluidine blue in 0.1 M sodium acetate buffer, pH 4.0, to visualize the anionic glycosaminoglycans followed by a 2-min counterstain in 0.1% (w/v) fast green FCF.
Haematoxylin and Eosin staining
Selected tissue sections were stained in Mayers Haematoxylin (5 min) to assess cellular morphology, rinsed in tap water blued in Scotts Blueing solution (1 min) and counterstained in 0.0001% eosin (5 min), dehydrated in 95% (v/v) ethanol then absolute ethanol, cleared in xylene and mounted.
Immunohistochemistry
Aggrecan and Versican were immunolocalised using a Sequenza vertical cover-plate immunostaining system [17-20]. Endogenous peroxidase activity was blocked by incubating the tissue sections with 3% H2O2 for 5 min and after washing in water non-specific binding sites were blocked with 10% swine serum for 10 min. The sections were pre-digested for 2h at 37°C with 0.05 U/ ml chondroitinase ABC prior to incubation with anti-versican 12C5 MAb (1/2000 dilution) or anti-aggrecan MAb Clone 969D411 (1/10,000 dilution) overnight at 4°C. The primary Ab's were subsequently localised using biotinylated anti-mouse IgG secondary antibody. Horse-radish peroxidase conjugated streptavidin was used to visualise the tissue immune complexes using Nova RED substrate for colour development. Negative control sections were also prepared where an irrelevant isotype matched mouse IgG directed against Aspergillus niger glucose oxidase, was substituted for authentic primary antibody. The stained tissue specimens were examined by bright field microscopy using a Leica photomicroscope linked to a DFC 480 digital camera.
Localisation of hyaluronan (HA) and hyaluronan binding protein (HABP)
HA and HABP were localised in a similar manner to that described earlier [21-23] using biotinylated HA oligosaccharide and a biotinylated aggrecan G1-G2-KS probe (bG1-G2-KS) was prepared by partial digestion of bovine nasal cartilage with testicular hyaluronidase, isolation and labelling as outlined earlier [21-23]. The avidin-HRP detection system was used to visualise the HA and HABP localisations using NovaRED as substrate. MAb 5-D-4 was also used to localise the bG1-G2-KS in tissue sections and rabbit anti mouse IgG HRP secondary antibody used for detection. Tissue sections destined for HA localisations were pre-treated for 8h at 37°C with Chondroitinase ABC (0.1 U/ml), keratanase-1 (K'ase-I) (0.05 U/ml), and keratanase-2 (K'ase-II) (0.5 mU/ml) in 0.1 M Tris acetate buffer (pH 6.5) to deplete the section of aggrecan prior to addition of bG1-G2-KS. In sections where HABPs were to be visualised the tissues received a pre-digestion step with bovine testicular hyaluronidase.
Localization of hyaluronan
HA was visualised by incubation of IVD tissue sections with biotinylated aggrecan G1-G2-KS HA binding link complex (5 µg/ ml in phosphate buffer pH 7.4 containing 1% BSA) overnight at 4°C. Colour development was undertaken with avidinbiotin- peroxidase complex and NovaRED substrate. MAb 5-D-4 (1/50,000 dilution) was also used to localise the KS component of the bG1-G2-KS in tissue sections and rabbit anti mouse IgG HRP secondary antibody used for detection. Control sections were also processed in which primary or secondary reagents were either omitted, or tissue sections were predigested with Streptomyces hyaluronidase to remove the endogenous HA.
Localisation of HABPs
Tissue sections were predigested with bovine testicular hyaluronidase (1 mg/ml) in 0.1 M sodium acetate buffer pH 5.3 containing 0.15 M NaCl for 1 h at 37°C, rinsed in TBS and the bHA oligosaccharide (2 µg/ml) in TBS was added for 1 h at 37°C. Avidin HRP conjugate (0.1 mg/ml) in 50 mM Tris-HCl buffer pH 7.2 was used for the detection step (1 h at room temperature) using NovaRED substrate. Negative control samples, did not receive the hyaluronidase pre-digestion step.
Results
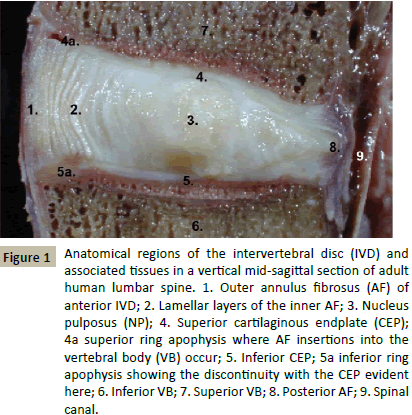
Figure 1 outlines the main anatomical regions of the normal adult human intervertebral disc and adjacent spinal tissues. The annular lamellae are well defined, the NP bulging evident in this vertical section indicates high aggrecan content. The ring apophysis on the outer margins of the CEP are also evident. These are the annular attachment points into the VBs and an area where Sharpeys fibres are found. The anterior longitudinal ligament is also evident extending past the IVD down the spine.
Figure 1: Anatomical regions of the intervertebral disc (IVD) and associated tissues in a vertical mid-sagittal section of adult human lumbar spine. 1. Outer annulus fibrosus (AF) of anterior IVD; 2. Lamellar layers of the inner AF; 3. Nucleus pulposus (NP); 4. Superior cartilaginous endplate (CEP); 4a superior ring apophysis where AF insertions into the vertebral body (VB) occur; 5. Inferior CEP; 5a inferior ring apophysis showing the discontinuity with the CEP evident here; 6. Inferior VB; 7. Superior VB; 8. Posterior AF; 9. Spinal canal.
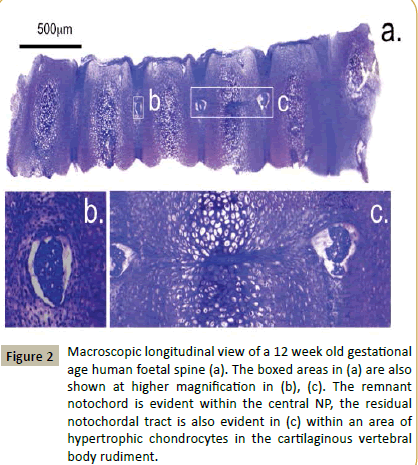
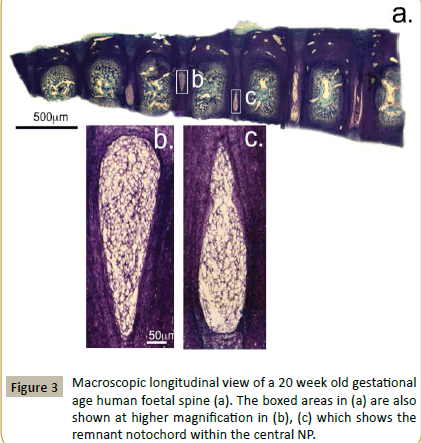
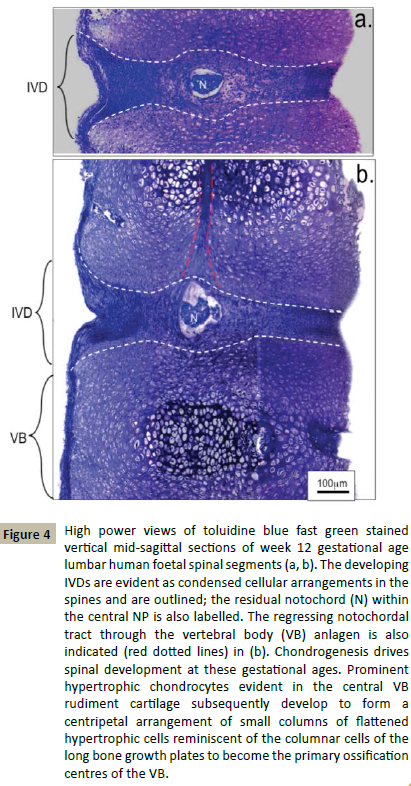
Macroscopic examination of longitudinal toluidine blue-fast green stained sections of a 12 and 20 week gestational age human foetal spine identified notochordal remnants (Figures 2 and 3). Examination of the cellular morphologies revealed condensations of smaller cells in the notochord at 12 weeks however these were less densely packed at 20 weeks in the developing IVD interspace with the lamellae of the AF becoming evident (Figure 4). Notochordal remnants were evident centrally in the developing NP containing densely packed notochordal cells. Some of the notochordal cells were vacuolated however they contained little surrounding ECM and the notochord was surrounded by a sheath. Evidence of notochordal tract regression longitudinally in the central VB was evident at 12 weeks (Figure 4b). The vertebral rudiment cartilages were highly cellular and contained cells of a chondrocytic morphology. By 12 weeks many of the VB cells were undergoing hypertrophy becoming arranged centripetally in the VB rudiment in regions destined to become the ossification centres. Chondrogenesis is the major driving force at this stage in foetal spinal development and there are at least four populations of cells of a differing size range and morphology.
Figure 2: Macroscopic longitudinal view of a 12 week old gestational age human foetal spine (a). The boxed areas in (a) are also shown at higher magnification in (b), (c). The remnant notochord is evident within the central NP, the residual notochordal tract is also evident in (c) within an area of hypertrophic chondrocytes in the cartilaginous vertebral body rudiment.
Figure 4: High power views of toluidine blue fast green stained vertical mid-sagittal sections of week 12 gestational age lumbar human foetal spinal segments (a, b). The developing IVDs are evident as condensed cellular arrangements in the spines and are outlined; the residual notochord (N) within the central NP is also labelled. The regressing notochordal tract through the vertebral body (VB) anlagen is also indicated (red dotted lines) in (b). Chondrogenesis drives spinal development at these gestational ages. Prominent hypertrophic chondrocytes evident in the central VB rudiment cartilage subsequently develop to form a centripetal arrangement of small columns of flattened hypertrophic cells reminiscent of the columnar cells of the long bone growth plates to become the primary ossification centres of the VB.
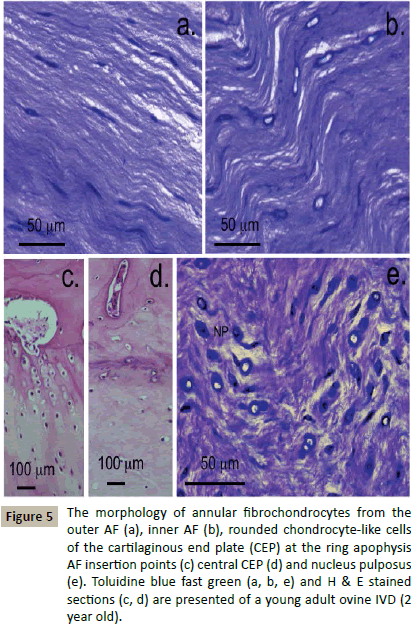
Definition of some of the cellular morphologies evident in a normal young adult ovine IVD (Figure 5). The cells in the AF have characteristic elongated fusiform morphologies and cellular extensions between adjacent cells forming short strings of communicating cells layed down along the annular collagen fibre bundles (Figure 5a). Inner cells of the AF were plumper than those of the outer AF however evidence of cellular interconnections were still evident (Figure 5b). The cells of the CEPs were rounded cells of a chondrocytic morphology, at the CEP margins the cells were arranged into small columns along the collagen fibres inserting into the VB at the ring apophysis (Figure 5c) whereas in the central CEP they were more uniformly distributed (Figure 5d). Cells in the NP had a rounded morphology and a dense pericellular matrix visualised by toluidine blue staining (Figure 5e).
Figure 5: The morphology of annular fibrochondrocytes from the outer AF (a), inner AF (b), rounded chondrocyte-like cells of the cartilaginous end plate (CEP) at the ring apophysis AF insertion points (c) central CEP (d) and nucleus pulposus (e). Toluidine blue fast green (a, b, e) and H & E stained sections (c, d) are presented of a young adult ovine IVD (2 year old).
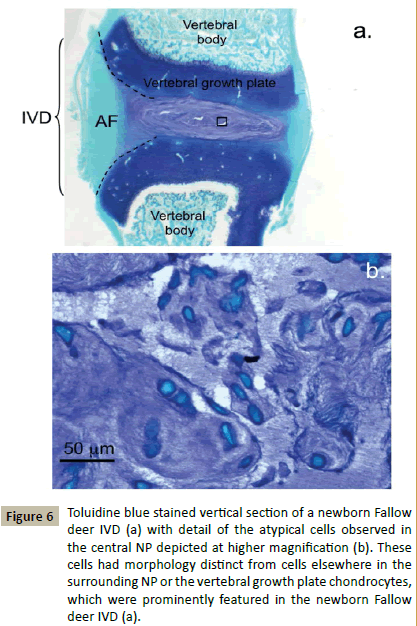
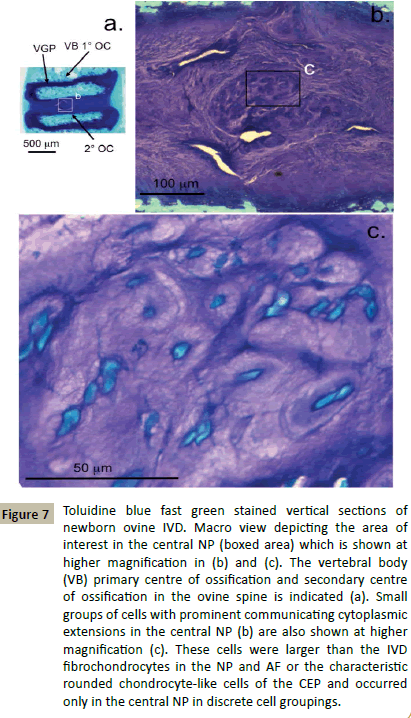
Examination of the spinal components and cellular morphologies evident in newborn deer demonstrated some interesting features. The vertebral growth plates were very prominent and highly cellular containing rounded chondrocytes in an ECM with a relatively high GAG content compared to the adjacent developing IVD (Figure 6a). The AF regions of the IVD were discernable but contained very little GAG, the inner AF lamellae were stained with toluidine blue. Closer examination of the central NP identified small groups of cells arranged in discrete bag-like structures which stained with toluidine blue (Figure 6b). These cells stained intensely with fast green counterstain, some had distorted shapes, many were interconnected with small extensions of their plasma membranes, their morphologies however were neither fibroblastic or chondrocytic (Figure 6b). Newborn ovine IVDs also displayed a prominent highly cellular vertebral growth plate rich in GAG however it was not as extensive as in the deer and it was also separated from the developing IVD by a secondary ossification centre (Figure 7a). Ovine newborn IVDs contained similar cellular arrangements in the central NP (Figure 7b and 7c) to those observed in the newborn deer IVD.
Figure 6: Toluidine blue stained vertical section of a newborn Fallow deer IVD (a) with detail of the atypical cells observed in the central NP depicted at higher magnification (b). These cells had morphology distinct from cells elsewhere in the surrounding NP or the vertebral growth plate chondrocytes, which were prominently featured in the newborn Fallow deer IVD (a).
Figure 7: Toluidine blue fast green stained vertical sections of newborn ovine IVD. Macro view depicting the area of interest in the central NP (boxed area) which is shown at higher magnification in (b) and (c). The vertebral body (VB) primary centre of ossification and secondary centre of ossification in the ovine spine is indicated (a). Small groups of cells with prominent communicating cytoplasmic extensions in the central NP (b) are also shown at higher magnification (c). These cells were larger than the IVD fibrochondrocytes in the NP and AF or the characteristic rounded chondrocyte-like cells of the CEP and occurred only in the central NP in discrete cell groupings.
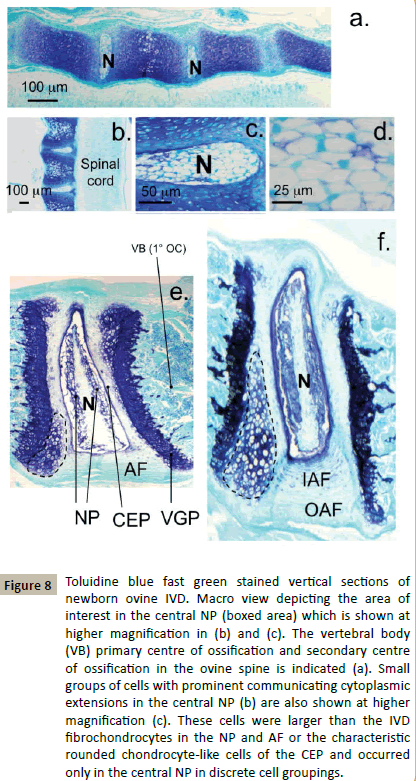
Examination of the cellular morphologies evident in mouse spinal development demonstrated some differences with the human, deer and sheep cellular populations. In foetal murine spines the notochord was a prominent feature occupying a large area of the developing IVD (Figure 8a). In the newborn murine spine the notochordal cells were not surrounded by an extensive ECM but were densely packed and extensively interconnected (Figure 8b and 8d). The notochord was displaced dorsally in the newborn murine spine towards the spinal canal and surrounded by a dense arrangement of chondrocyte like cells in a GAG rich ECM (Figure 8c). Hypertrophic cells in the developing VB segments was also evident in the newborn murine spine (Figure 8b). At 3 weeks the murine VGPs were prominent with small columns of flattened hypertrophic cells observed, the notochord was prominent within the NP but flattened (Figure 8e). The NP was defined around the notochord with GAG layed down around the prominent larger chondrocyte like cells surrounding the notochord (Figure 8e). The CEP contained cells of chondrocyte-like morphology. An additional population of prominent densely packed large cells was evident on the margins of the VGP with the CEP (Figure 8e, dotted lines). These may represent a progenitor cell population. At 8 weeks the notochord was still flattened and highly cellular in the murine spine (Figure 8f). The NP cells surrounding the notochord however had a prominent GAG rich ECM and the CEP was better defined than at 3 weeks. The VGPs and associated progenitor cell population were even more prominent at 8 weeks, GAG layed down in the annular arcades in the inner AF aided in the identification of the AF cells (Figure 8f).
Figure 8: Toluidine blue fast green stained vertical sections of newborn ovine IVD. Macro view depicting the area of interest in the central NP (boxed area) which is shown at higher magnification in (b) and (c). The vertebral body (VB) primary centre of ossification and secondary centre of ossification in the ovine spine is indicated (a). Small groups of cells with prominent communicating cytoplasmic extensions in the central NP (b) are also shown at higher magnification (c). These cells were larger than the IVD fibrochondrocytes in the NP and AF or the characteristic rounded chondrocyte-like cells of the CEP and occurred only in the central NP in discrete cell groupings.
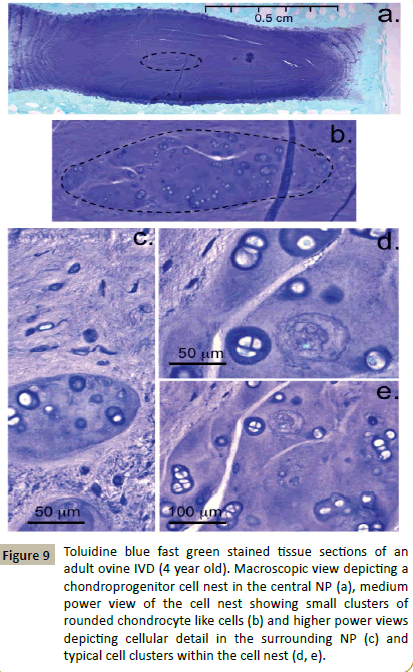
Close examination of the ovine spines from the 12, 18 and 48 month sample groups showed that all IVDs examined contained typical fusiform fibroblastic cells in the annular layers, rounded fibrochondrocytes in the NP and rounded chondrocyte-like cells in the CEPs. A further population of cells of dissimilar morphology to these aforementioned cell types was also identified in 63 of the 72 IVDs examined (Figure 9). These cells also had a rounded morphology but were larger than the other IVD cell types and a significant proportion of these cells were undergoing division (Figure 9d and 9e). These chondroid cell nests were confined within bag-like structures with defined edges demarcating them from the surrounding NP (Figure 9b). The surrounding NP matrix had discernable ECM fibrillar material in the toluidine blue stained tissue sections however the aforementioned cell nests were surrounded by an ECM devoid of such fibrillar material (Figure 9c). The cells within such cell nests were surrounded by a well defined basophilic pericellular matrix which stained positively with toluidine blue (Figure 9d and 9e).
Figure 9: Toluidine blue fast green stained tissue sections of an adult ovine IVD (4 year old). Macroscopic view depicting a chondroprogenitor cell nest in the central NP (a), medium power view of the cell nest showing small clusters of rounded chondrocyte like cells (b) and higher power views depicting cellular detail in the surrounding NP (c) and typical cell clusters within the cell nest (d, e).
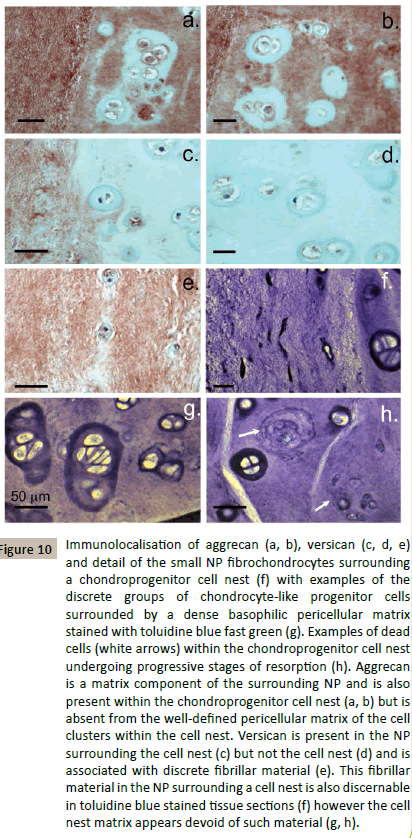
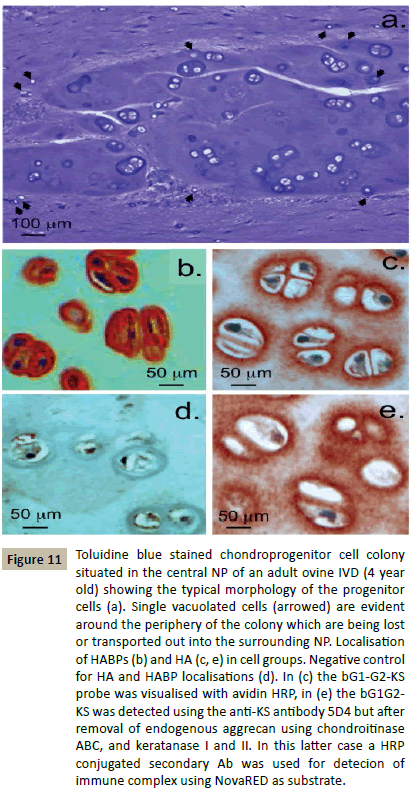
Immunolocalisation of aggrecan and versican, two major CSproteoglycans of the IVD ECM showed that the chondroid cell nests identified in Figure 7 also contained aggrecan (Figure 10a and 1b) but not versican (Figure 10c and 10d). Versican was an ECM proteoglycan of the surrounding NP (Figure 10d). The pericellular matrices surrounding the discrete groups of cells in the chondroid cell nests did not contain aggrecan (Figure 10a and 10b). The cells in the chondroid cell nests were noticeably larger than the NP cells in the surrounding tissue (Figure 10f). Many of the cells in such chondroid cell nests were undergoing cell division (Figure 10g). A few dead cells and residual organelles and vesicles from resorption of a dead cell were also detected in some cell nests (Figure 10h). Small cells containing small vesicles occurred around the periphery of the chondroid cell colonies (Figure 11a) these appeared to be escaping or were being transported out of the chondroid cell mass. Localisation of HABPs and HA in the chondroid cell colonies showed that the prominent pericellular matrix surrounding these cells contained cell associated HABPs, intracellular HABPs were also detected (Figure 11b). HA had a more diffuse distribution around these cells (Figure 11c and 11e).
Figure 10: Immunolocalisation of aggrecan (a, b), versican (c, d, e) and detail of the small NP fibrochondrocytes surrounding a chondroprogenitor cell nest (f) with examples of the discrete groups of chondrocyte-like progenitor cells surrounded by a dense basophilic pericellular matrix stained with toluidine blue fast green (g). Examples of dead cells (white arrows) within the chondroprogenitor cell nest undergoing progressive stages of resorption (h). Aggrecan is a matrix component of the surrounding NP and is also present within the chondroprogenitor cell nest (a, b) but is absent from the well-defined pericellular matrix of the cell clusters within the cell nest. Versican is present in the NP surrounding the cell nest (c) but not the cell nest (d) and is associated with discrete fibrillar material (e). This fibrillar material in the NP surrounding a cell nest is also discernable in toluidine blue stained tissue sections (f) however the cell nest matrix appears devoid of such material (g, h).
Figure 11: Toluidine blue stained chondroprogenitor cell colony situated in the central NP of an adult ovine IVD (4 year old) showing the typical morphology of the progenitor cells (a). Single vacuolated cells (arrowed) are evident around the periphery of the colony which are being lost or transported out into the surrounding NP. Localisation of HABPs (b) and HA (c, e) in cell groups. Negative control for HA and HABP localisations (d). In (c) the bG1-G2-KS probe was visualised with avidin HRP, in (e) the bG1G2- KS was detected using the anti-KS antibody 5D4 but after removal of endogenous aggrecan using chondroitinase ABC, and keratanase I and II. In this latter case a HRP conjugated secondary Ab was used for detecion of immune complex using NovaRED as substrate.
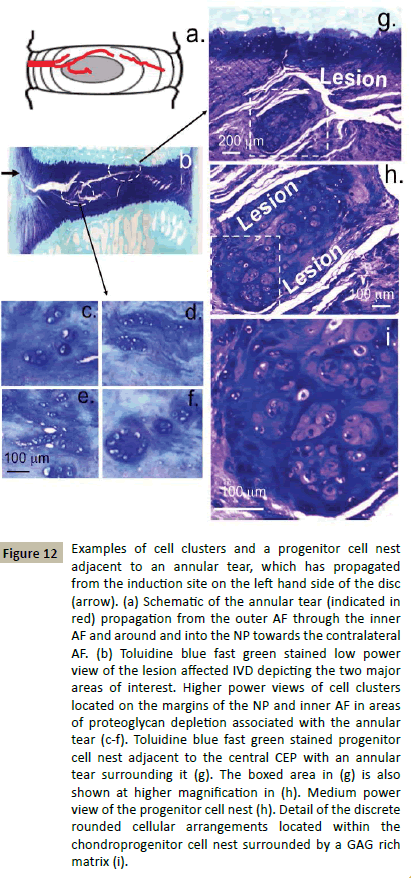
In the course of our sheep work on the large annular lesion model of experimental disc degeneration we have collected a histology archive of the cellular populations associated with areas of the IVD where the lesion has entered or in areas where remodelling consistent with an attempted repair response had occurred (Figure 12a and 12b). Examination of this histology archive identified small groups of rounded cells in the vicinity of a lesion which had entered into the inner AF (Figure 12c and 12f) and a chondroid clone of rounded cells surrounded by an annular lesion which had propagated around them in the NP close to the central CEP (Figure 12g and 12i). The cells in the chondroid colony were arranged into small discrete arrangements of cells closely grouped together, these were surrounded by a dense GAG matrix (Figure 12i). Such cells may represent an adult progenitor cell population which had yet to be released to migrate to defect sites to effect repair processes (Figure 12c and 12f). Many of the cellular arrangements identified in Figure 12 resembled those previously identified in adult chondroid cell nests in adult ovine IVDs (Figures 9-11).
Figure 12: Examples of cell clusters and a progenitor cell nest adjacent to an annular tear, which has propagated from the induction site on the left hand side of the disc (arrow). (a) Schematic of the annular tear (indicated in red) propagation from the outer AF through the inner AF and around and into the NP towards the contralateral AF. (b) Toluidine blue fast green stained low power view of the lesion affected IVD depicting the two major areas of interest. Higher power views of cell clusters located on the margins of the NP and inner AF in areas of proteoglycan depletion associated with the annular tear (c-f). Toluidine blue fast green stained progenitor cell nest adjacent to the central CEP with an annular tear surrounding it (g). The boxed area in (g) is also shown at higher magnification in (h). Medium power view of the progenitor cell nest (h). Detail of the discrete rounded cellular arrangements located within the chondroprogenitor cell nest surrounded by a GAG rich matrix (i).
Discussion
In the present study, we detected cell clusters of a similar morphology to NP cells recently proposed to be a progenitor cell population in the human IVD (12). These cells were evident in adult ovine IVDs (1-4 years old) enclosed in a capsule-like structure within the NP. Aggrecan was immunolocalised both in the surrounding NP and within these capsules. Versican was not localised within these structures but was detected in the surrounding NP, consistent with its identity as a marker of fibrocartilaginous tissue. Similar, enclosed, smaller groups of cells were also observed in the newborn ovine and cervine NP however these had a differing morphology to the notochordal cells of the murine IVD. Notochordal cells are reported to have a physalipherous morphology with a bubbly or vacuolated cytoplasm containing large intracytoplasmic droplets of mucoid material. Results presented in the present study question this generalisation. The chondroid cells observed in the present study had a similar morphology to human IVD progenitor cells (12) and to clustered notochordal cells previously described [24-27]. Cells within these cell clusters were undergoing cell division, consistent with their identity as a progenitor cell population contained within a hyaline-like chondroid matrix but not typical of mature IVD cells which rarely undergo cell division. The capsule around these cells in the central NP may have a mechano-protective role. While small enclosed groups of cells were observed in the newborn ovine and cervine IVD they had a dissimilar morphology to these adult cell nests but may be a less mature form of these entities. The notochordal cell is responsive to its weighted environment and this may be the driving force for maturational changes in these progenitor cell nests with the physical properties (stiffness, elasticity) of the matrix surrounding progenitor/stem cells directing their differentiation in-vitro [28,29].
matricryptin ECM fragments, gradients of ECM molecules layed down by pericellular matrix proteoglycans such as perlecan and growth factors from the FGF and TGF-β families promote stem cell migration to sites of tissue damage, cell lineage development and stem/progenitor cell proliferation. In the present study we also showed that HABPs were localised intracellularly and on the progenitor cell plasma membranes. Cell surface (CD-44, RHAMM/ IHABP, TLR-2, TLR-4) and intracellular (HABP4, IHABP, HABP1/ p32/C1qBP) HABP members have been identified in intracellular organelles such as the mitochondria (HABP1), nucleus and nucleoli (RHAMM/IHABP) and in cytoplasmic vesicles. HABPs have important cell-signalling properties and can bind HA preventing oxidative damage. HABPs also have interactive properties with cytoskeletal proteins (Talin, ezrin, moesin, radixin), influencing actin and tubulin microdynamics controlling cell signalling, regulating cell shape and migratory properties and assembly of mitotic spindle formations with attendant effects on cell proliferation. CD-44 has roles in the stabilisation of the HA coats of many cell types [30].
Loss of notochordal cells has been observed in a needle stick disc degeneration model in mice [32]. IVD degeneration was accompanied by a shift in the NP cell notochordal phenotype to chondrocytic and fibrochondrocytic forms suggesting that the notochordal cell population in the mouse may act as a cell reserve or a progenitor cell niche which regulates tissue homeostasis and promotes IVD repair [32].
The progenitor cell nests we observed in the adult ovine IVD occupied a similar central location in the NP to the notochordal cells described in mouse IVDs. The cells within progenitor cell niches in tissues are generally a quiescent or slowly proliferating cell type until a stimulus for them to differentiate occurs such as a change in their biomechanical microenvironment in the event of an injury or some other directive cue from an ECM component. If the chondroid cell nests observed in the present study are an adult stem cell niche this would be advantageous for IVD homeostasis. When activated, these committed NP cells could differentiate and synthesise the correct ECM required for NP repair, a primary area of importance in IVD degeneration. This has prompted the search for endogenous progenitor cells in the IVD and their therapeutic application for the preservation and maintenance of IVD homeostasis. Evidence from the present study indicates that the chondroid cell nests identified may well represent a progenitor cell population, similar cell populations have also been identified in the human IVD [12]. We also observed small vacuolated cells at the periphery of these progenitor cell colonies about to be released into the surrounding NP, these may be replacement cells for the resident NP cells which have undergone cell death through apoptosis, senescence or autophagy. A protective role for these chondroprogenitor colonies is also suggested from our findings in an ovine large annular lesion model of IVD degeneration [33]. Examination of IVDs where degeneration had been induced by a controlled outer annular lesion allowed us to identify small reactive clusters of cells around the annular lesions which penetrated into the inner AF in areas depleted of proteoglycan [33]. Mechanostransductive signals arising from altered biomechanical cues to such cells may have initiated cell division as part of an attempted repair response, cell division is not a normal response in inner AF cells. We also observed discrete NP cell nests enclosed in a well-defined colony adjacent to an annular lesion with some evidence of release of small vacuolated cells from the its margins presumably to effect repair of the lesion. Culture of individual NP cells and cell clusters from such colonies in human IVDs demonstrated stem cell markers [12]. Significantly, the single cells displayed a greater proliferative capacity than the clustered cell arrangements however if these clustered cells were similar to the cell nests observed in the present study surrounded by HA then this may have inhibited their proliferative properties. As the disc degenerates and loses proteoglycan its weight bearing properties are compromised and changes in the local biomechanical microenvironment of progenitor cell colonies may direct stem cell differentiation in-situ ([34-36]. Progenitor cells are responsive to forces they experience in-vitro and invivo, a better understanding of these biomechemical cues and the molecular signals that control progenitor cell differentiation and proliferation in-situ will be invaluable in future therapeutic applications.
Considerable evidence now exists that notochordal cells are beneficial in NP repair, they produce more proteoglycans in culture than the small resident NP cells and when co-cultured with NP cells they assume a directing role, up-regulating production of ECM components [37-40] and also protect the small NP cells from IL-1 induced cell death [41]. Thus the progenitor cells observed in the present study are certainly worthy of further investigation.
The use of the resident progenitor stem cells for repair of the degenerate IVD is an appealing proposal however major technical obstacles still need to be overcome before this may become a therapeutic reality. Currently, LinkN is the only biological treatment that can delay disc degeneration. Initial findings using intradiscal bone marrow derived stem cells in laboratory based pre-clinical studies and in clinical trials have also been very promising [35,36,42-46]. The NP progenitor cells examined in the present study may represent the ideal cell type for therapeutic purposes. The question of how the stem cells are activated in-situ, their proliferation, differentiation and development of appropriate cell lineages for repair purposes still remain to be resolved [47-50]. How the HA pericellular coat around the progenitor cell nests is dismantled in the IVD also remains to be determined. The hypertrophic cells of the growth plate are also surrounded by a pericellular matrix of condensed HA however this disappears when the growth plate undergoes maturational changes during endochondral bone formation. Oocytes are also surrounded by an extensive HA coat which is depolymerised post-fertilization this is a relatively poorly researched area of ECM remodeling however with scant details available on how this actually occurs. The human genome contains 6 hyaluronidase-like genes Hyal1, Hyal2, Hyal3, Hyal4, HyalP1 and PH20 [51]. HyalP1 is an inactive pseudogene and PH20 is a testicular enzyme. The remaining enzymes are expressed in cartilage and bone however, Hyal1 and Hyal2 are the major hyaluronidases. These act as N-acetyl hexosaminidases cleaving CS with lower efficiency than HA. Hyal1 is a lysosomal enzyme while Has2 is a GPI anchored enzyme on the outer surface of the plasma membrane. Hyal2 may initiate the degradation of HA into fragments which are subsequently endocytosed for further intracellular breakdown by Hyal1. HA oligosaccharides released from this HA depolymerisation step can also synergize with VEGF and promote angiogenesis during vascular invasion of the calcified growth plate leading to bone formation. HA oligosaccharides also promote MMP synthesis and activation and the synthesis of selected ECM components such as type I collagen thus they also have roles to play in ECM remodeling. Glycan-degrading enzymes are emerging as agents which promote ECM remodeling and wound repair [52,53]. Heparanase 1 and 2 expression are elevated in disc degeneration and promote angiogenic wound repair [54]. Heparanase 1 and 2 are produced by osteoblasts these degrade growth plate perlecan releasing HS bound growth factors such as FGF-2 which promote angiogenic processes and bone formation [55,56]. Future research may delineate further the role of glycan depolymerising enzymes in ECM remodeling in health and disease.
How the activated stem cell attains migratory properties and homes to tissue defects is also still an unresolved technical issue. Changes in the intrinsic biomechanical stresses to the stem cells may be one factor which contributes to stem cell activation, stem cells are known to be influenced by extrinsic forces [28,31] and there is also evidence from our ovine annular lesion model of IVD degeneration of this possibility [34], disc degeneration has also been shown to alter the properties of the resident NP progenitor cells [34]. The ECM molecules which tether the stem cells are also important determinants of their differentiation [57] and migratory properties [58]. Migratory stem cells also express stromal cell derived factor -1(SDF-1) and its specific binding partner, chemokine (C-X-C motif) receptor-4 (CXCR4). These have potent chemotactic activity and their expression is upregulated in the degenerate IVD [59], moreover intradiscal administration of SDF-1/CXCR4 over-expressing stem cells promotes repair of degenerate IVDs [60]. SDF-1/CXCR4 mediated cell signalling promotes digit regeneration in mice a process driven by BMP-2 [61,62].
Many ECM components undergo limited enzymatic cleavages during the turnover of connective tissues resulting in the release of bioactive fragments of ECM components (matrikines) or exposure of bioactive cryptic epitopes (matricryptins) on ECM components [63]. The bioactivities displayed by the Matricryptins and Matrikines normally differ markedly from those of the native molecules from which they are derived leading to the release of a diverse array of bioactivities which regulate an extremely diverse range of physiological processes including wound repair and ECM remodeling [63]. Many of the released fragments of ECM molecules stimulate MMP and ADAMTS production, nitric oxide, chemokines and cytokines. Considerable interest exists around the identification of matricryptins to stimulate tissue repair. Cryptic peptides [64] which recruit resident progenitor cells to sites of tissue damage to effect ECM/remodelling to promote repair processes have been described [65] and of relevance to repair of the degenerate IVD. Link-N, a cryptic fragment of linkprotein has been applied to a number of IVD studies and shown to stimulate the resident IVD cell populations as well as progenitor stem cells to upregulate production of collagen and aggrecan and stimulate repair of the degenerate IVD [66-72]. Future studies will hopefully uncover basic data applicable to the application of the resident progenitor stem cell population we have observed as a therapeutic cell type.
Acknowledgements
This study was funded by NHMRC Project Grant 352562. Ms Susan Smith and Prof Christopher B Little Raymond Purves Laboratory, Kolling Institute, University of Sydney are thanked for undertaking the histology presented in this study and supply of mouse tissue sections respectively.
References
- Shapiro I, Risbud MV (2014)Introduction to the structure, Function and Comparative Anatomy of the vertebrae and the intervertebral disc. In: Shapiro I, Risbud MV(Eds). The Intervertebral disc Molecular and structural studies of the disc in health and disease Vienna: Springer-Verlag 3-16.
- Humzah MD, Soames RW (1988) Human intervertebral disc: structure and function. Anat Rec 220: 337-356.
- Cortes D, Elliott DM (2014) The intervertebral disc: overview of disc mechanics In: Shapiro I, Risbud MV (Eds). The Intervertebral disc Molecular and structural studies of the disc in health and disease. Vienna: Springer-Verlag17-31.
- Fleming A, Keynes RJ, Tannahill D (2001) The role of the notochord in vertebral column formation. J Anat 199: 177-180.
- Rodrigues-Pinto R, Richardson SM, Hoyland JA (2014)An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur Spine J 23: 1803-1814.
- Babic MS (1991) Development of the notochord in normal and malformed human embryos and fetuses. Int J Dev Biol 35:345-352.
- Risbud MV, Shapiro IM (2011) Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr 21: 29-41.
- McCann MR, Tamplin OJ, Rossant J, Séguin CA (2012) Tracing notochord-derived cells using a Noto-cre mouse: implications for intervertebral disc development. Dis Model Mech 5: 73-82.
- Choi KS, Cohn MJ, Harfe BD (2008) Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn 237: 3953-3958.
- Hunter CJ, Matyas JR, Duncan NA (2003)The three-dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J Anat 202: 279-291.
- Hunter CJ, Matyas JR, Duncan NA (2004) Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat 205: 357-362.
- Turner S, Balain B, Caterson B, Morgan C, Roberts S (2014) Viability, growth kinetics and stem cell markers of single and clustered cells in human intervertebral discs: implications for regenerative therapies. Eur Spine J 23:2462-2472.
- Kim JH, Deasy BM, Seo HY, Studer RK, Vo NV, et al. (2009) Differentiation of intervertebral notochordal cells through live automated cell imaging system in vitro. Spine (Phila Pa 1976) 34: 2486-2493.
- Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, et al. (2008) Are animal models useful for studying human disc disorders/degeneration? Eur Spine J 17: 2-19.
- Henriksson H, Thornemo M, Karlsson C, Hägg O, Junevik K, et al. (2009) Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine 34: 2278-2287.
- Henriksson HB, Svala E, Skioldebrand E, Lindahl A, Brisby H (2012) Support of concept that migrating progenitor cells from stem cell niches contribute to normal regeneration of the adult mammal intervertebral disc: a descriptive study in the New Zealand white rabbit. Spine 37:722-32.
- Melrose J, Smith S, Cake M, Read R, Perlecan W (2005) displays variable spatial and temporal immunolocalisation patterns in the articular and growth plate cartilages of the ovine stifle joint. Histochem Cell Biol 123:561-571.
- Melrose J, Smith S, Ghosh P, Perlecan W (2003) the multidomainheparan sulfate proteoglycan of basement membranes, is also a prominent component of the cartilaginous primordia in the developing human fetal spine. JHistochemCytochem 51:1331-1341.
- Melrose J, Smith S, Knox S, Perlecan W (2002) the multidomain HS-proteoglycan of basement membranes, is a prominent pericellular component of ovine hypertrophic vertebral growth plate and cartilaginous endplate chondrocytes. Histochem Cell Biol 118:269-280.
- Melrose J, Smith S, Whitelock J (2004) Perlecan immunolocalises to perichondral vessels and canals in human foetalcartilagenouspromordia in early vascular and matrix remodelling events associated with diarthrodial-joint development. J HistochemCytochem52:1405-1413.
- Melrose J, Smith S (2002)Histochemical visualization of the cartilage hyaladherins using a biotinylated hyaluronan oligosaccharide biotinylated aggrecan G1-link complex and biotinylated hyaluronan oligosaccharides. Histochem Cell Biol 117:327-333.
- Melrose J, Tammi M, Smith S (2004) Visualisation of hyaluronan and hyaluronan-binding proteins within ovine vertebral cartilages using bioaffinity probe. Methods Mol Med 101:65-78.
- Brisby H, Papadimitriou N, Brantsing C, Bergh P, Lindahl A, et al. (2013)The presence of local mesenchymal progenitor cells in human degenerated intervertebral discs and possibilities to influence these in vitro: a descriptive study in humans. Stem Cells Dev 22:804-814.
- Chan WC, Au TY, Tam V, Cheah KS, Chan D (2014) Coming together is a beginning: the making of an intervertebral disc. Birth Defects Res C Embryo Today 102: 83-100.
- Hunter CJ, Matyas JR, Duncan NA (2004) The functional significance of cell clusters in the notochordal nucleus pulposus: survival and signaling in the canine intervertebral disc. Spine 29: 1099-1104.
- Johnson WE, Eisenstein SM, Roberts S (2001) Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res 42: 197-207.
- Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126: 677-689.
- Melrose J, Tammi M, Smith S (2004) Visualisation of hyaluronan and hyaluronan-binding proteins within ovine vertebral cartilages using bioaffinity probe. Methods Mol Med 101:65-78.
- Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, et al. (2009) Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5: 17-26.
- Qu C, Rilla K, Tammi R, Tammi M, Kroger H, et al. (2014) Extensive CD44-dependent hyaluronan coats on human bone marrow-derived mesenchymal stem cells produced by hyaluronan synthases HAS1, HAS2 and HAS3. Int J Biochem Cell Biol 48:45-54.
- Solis MA, Chen YH, Wong TY, Bittencourt VZ, Lin YC, et al. (2012) Hyaluronan regulates cell behavior: a potential niche matrix for stem cells. Biochem Res Int 346-972.
- Yang F, Leung VY, Luk KD, Chan D, Cheung KM (2009) Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J Pathol 218:113-121.
- Melrose J, Shu C, Young C, Ho R, Smith MM (2012) Mechanical destabilization induced by controlled annular incision of the intervertebral disc dysregulates metalloproteinase expression and induces disc degeneration. Spine 37: 18-25
- Mizrahi O, Sheyn D, Tawackoli W, Ben-David S, Su S, et al. (2013) Nucleus pulposus degeneration alters properties of resident progenitor cells. Spine J 13: 803-814.
- Leung VY, Chan D, Cheung KM (2006) Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction. Eur Spine J 15 Suppl 3: 406-413.
- Yang F, Leung VY, Luk KD, Chan D, Cheung KM (2009) Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol Ther 17:1959-1966.
- Aguiar DJ, Johnson SL, Oegema TR (1999) Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res 246: 129-137.
- Erwin WM (2008) The Notochord, Notochordal cell and CTGF/CCN-2: ongoing activity from development through maturation. J Cell Commun Signal 2: 59-65.
- Erwin WM, Ashman K, O'Donnel P, Inman RD (2006) Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum 54:3859-3867.
- Erwin WM, Las Heras F, Islam D, Fehlings MG, Inman RD (2011) The regenerative capacity of the notochordal cell: tissue constructs generated in vitro under hypoxic conditions. J Neurosurg Spine 10:513-521.
- Erwin WM, Islam D, Inman RD, Fehlings MG, Tsui FW (2011) Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther 13: 215.
- Orozco L, Soler R, Morera C, Alberca M, Sánchez A, et al. (2011) Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 92: 822-828.
- Freeman BJ, Kuliwaba JS, Jones CF, Shu CC, Colloca CJ, et al. (2016) Allogeneic Mesenchymal Stem Cells Promote Healing in Postero-Lateral Annular Lesions and Improve Indices of Lumbar Intervertebral Disc Degeneration in an Ovine Model. Spine.
- Mulaibrahimovic A, Gronthos S, Zannettino AC, Howell S (2016) Allogeneic Mesenchymal Stem Cells Promote Healing in Postero-Lateral Annular Lesions and Improve Indices of Lumbar Intervertebral Disc Degeneration in an Ovine Model. Spine.
- Zeckser J, Wolff M, Tucker J, Goodwin J (2016) Multipotent Mesenchymal Stem Cell Treatment for Discogenic Low Back Pain and Disc Degeneration. Stem Cells Int: 3908389.
- Clarke LE, Richardson SM, Hoyland JA (2015) Harnessing the Potential of Mesenchymal Stem Cells for IVD Regeneration.Curr Stem Cell Res Ther 10: 296-306.
- Sivakamasundari V, Lufkin T (2013) Stemming the Degeneration: IVD Stem Cells and Stem Cell Regenerative Therapy for Degenerative Disc Disease. Adv Stem Cells.
- Wang F, Shi R, Cai F (2015) Stem Cell Approaches to Intervertebral Disc Regeneration: Obstacles from the Disc Microenvironment. Stem Cells Dev 24: 2479-2495.
- Li Z, Peroglio M, Alini M, Grad S (2015) Potential and limitations of intervertebral disc endogenous repair. Curr Stem Cell Res Ther 10: 329-338.
- Sakai D, Andersson G (2015) Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol 11: 243-256.
- Jandial R, Aryan HE, Park J, Taylor WT, Snyder EY (2008) Stem cell-mediated regeneration of the intervertebral disc: cellular and molecular challenge. Neurosurg Focus 24: 21.
- Csoka AB, Frost GI, Stern R (2001) The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol 20: 499-508.
- Nasser NJ (2008) Heparanase involvement in physiology and disease. Cell Mol Life Sci 65: 1706-1715.
- Gingis-Velitski S, Ishai-Michaeli R, Vlodavsky I, Ilan N (2007) Anti-heparanase monoclonal antibody enhances heparanase enzymatic activity and facilitates wound healing. FASEB J 21:3986-3893.
- Rodrigues LM, Theodoro TR, Matos LL, Mader AM, Milani C, et al. (2011) Heparanase isoform expression and extracellular matrix remodeling in intervertebral disc degenerative disease. Clinics 66:903-909.
- Zcharia E, Zilka R, Yaar A, Yacoby-Zeevi O, Zetser A, et al. (2005) Heparanase accelerates wound angiogenesis and wound healing in mouse and rat models. FASEB J 19: 211-221.
- Kram V, Zcharia E, Yacoby-Zeevi O, Metzger S, Chajek-Shaul T, et al. (2006) Heparanase is expressed in osteoblastic cells and stimulates bone formation and bone mass. J Cell Physiol 207: 784-792.
- Trappmann B1, Gautrot JE, Connelly JT, Strange DG, Li y, et al. (2012) Extracellular-matrix tethering regulates stem-cell fate. Nature Materials 11: 642-649.
- Henriksson HB, Papadimitriou N, Tschernitz S, Svala E, Skioldebrand E, et al. (2015) Indications of that migration of stem cells is influenced by the extra cellular matrix architecture in the mammalian intervertebral disk region. Tissue Cell 47: 439-455.
- Zhang H, Zhang L, Chen L, Li W, Li F, Chen Q (2014)Stromal cell-derived factor-1 and its receptor CXCR4 are upregulated expression in degenerated intervertebral discs. Int J Med Sci 11:240-245.
- Wei JN, Cai F, Wang F, Wu XT, Liu L (2016) Transplantation of CXCR4 Overexpressed Mesenchymal Stem Cells Augments Regeneration in Degenerated Intervertebral Discs.
- Lee J, Marrero L, Yu L, Dawson LA, Muneoka K, et al. (2013) SDF-1α/CXCR4 signaling mediates digit tip regeneration promoted by BMP-2. Dev Biol 382: 98-109.
- Ricard-Blum S, Salza R (2014) Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Exp Dermatol 23: 457-463.
- VineetAgrawal, Scott A. Johnson, Janet Reing, Li Zhang (2010) Epimorphic regeneration approach to tissue replacement in adult mammals Proc Natl Acad Sci107: 3351-3355.
- Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL (2011) Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature 476: 409-413.
- Mwale F, Wang HT, Roughley P, Antoniou J, Haglund L (2014) Link N and mesenchymal stem cells can induce regeneration of the early degenerate intervertebral disc. Tissue Eng Part A 20: 2942-2949.
- Mwale F, Masuda K, Pichika R, Epure LM, Yoshikawa T, et al. (2011) The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res Ther 13: R120.
- Gawri R, Antoniou J, Ouellet J, Awwad W, Steffen T, et al. (2013) Best paper NASS 2013: link-N can stimulate proteoglycan synthesis in the degenerated human intervertebral discs. Eur Cell Mater 26: 107-119.
- Wang Z, Weitzmann MN, Sangadala S, Hutton WC, Yoon ST (2013) Link protein N-terminal peptide binds to bone morphogenetic protein (BMP) type II receptor and drives matrix protein expression in rabbit intervertebral disc cells. J Biol Chem 288: 28243-28253.
- Wang Z, Hutton WC, Yoon ST (2013) ISSLS Prize winner: Effect of link protein peptide on human intervertebral disc cells. Spine 38: 1501-1507.
- Antoniou J, Wang HT, Alaseem AM, Haglund L, Roughley PJ, et al. (2012) The effect of Link N on differentiation of human bone marrow-derived mesenchymal stem cells. Arthritis Res Ther 14: R267.
- Petit A, Yao G, Rowas SA, Gawri R, Epure L, et al. (2011) Effect of synthetic link N peptide on the expression of type I and type II collagens in human intervertebral disc cells.Tissue Eng Part A 17: 899-904.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences